Fig.1. Hene synthesis and iron metabolism in erythroid cells.
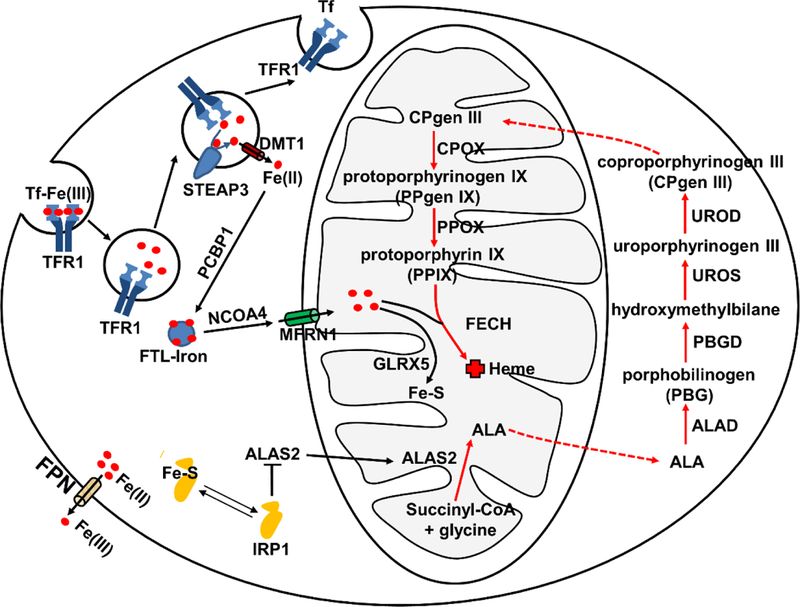
Iron acquisition in erythroid cells is dependent on endocytosis of Tf-bound Fe (Tf-Fe(III)) via the transferrin receptor (TFR1). Iron (Fe(III)) is imported into the cytoplasm by DMT1 after reduction by STEAP3. Iron can be stored in the ferritin (FTL) (FTL-Iron) mediated by PCBP1 and can be released from FTL promoted by NCOA4. MFRN1 is responsible for bringing iron to the mitochondria for heme synthesis. The red arrow represents overall heme synthetic pathway. Heme biosynthesis initiates in the mitochondrial matrix and is catalyzed by δ-aminolevulinic acid synthase (ALAS2) to synthesize δ-aminolevulinic acid (ALA) from glycine and succinyl-coenzyme A. ALA is subsequently transported out of the mitochondria to the cytosol for the following four enzymatic reaction steps: ALA to porphobilinogen (PBG) catalyzed by ALA dehydratase (ALAD); PBG to an unstable polymer hydroxymethylbilane by porphobilinogen deaminase (PBGD); hydroxymethylbilane to uroporphyrinogen III (UROgen III) by uroporphyrinogen synthase (UROS); and UROgen III to coproporphyrinogen III (CPgen III) by uroporphyrinogen decarboxylase (UROD). CPgen III is then transported into the mitochondria where coproporphyrinogen oxidase (CPOX), a mitochondrial intermembrane space enzyme, catalyzes the formation of protoporphyrinogen IX (PPgen IX). The inner mitochondrial membrane enzyme protoporphyrinogen oxidase (PPOX) catalyzes the formation of protoporphyrin IX (PPIX) from PPgen IX in the mitochondrial matrix. On the last step, ferrochelatase (FECH) catalyzes the insertion of ferrous iron (Fe2+) into PPIX to form heme. Iron is also used for Fe-S cluster synthesis with involvement of GLRX5. IRP1 binding can inhibit translation of ALAS2 to prevent the accumulation of toxic heme intermediates. Cellular iron efflux is mediated by FPN and requires iron oxidation on the extracellular side.
