Abstract
In recent years, a significant number of studies have investigated the preventive role of vitamin D in a number of different neoplasms. In this study, we analyze various components of the vitamin D signaling pathways in the human uveal tract and uveal melanoma, including analysis of the expression of vitamin D receptors (VDR), the activating and inactivating hydroxylases, respectively, CYP27B1 and CYP24A1, and the retinoic acid-related orphan receptors (ROR) α (RORα) and γ (RORγ) in these tissues. We further analyzed the expression of VDR, CYP27B1, CYP24A1, and ROR in relation to melanin levels, clinical stage and prognosis. Our study indicated that the uveal melanoma melanin level inversely correlated with VDR expression. We further showed that vitamin D is metabolized in uveal melanoma. This is significant because until now there has been no paper published, that would describe presence of VDR, hydroxylases CYP27B1 and CYP24A1, and RORα and RORγ in the human uveal tract and uveal melanomas. The outcomes of our research can contribute to the development of new diagnostic and therapeutic methods in uveal tract disorders, especially in uveal melanoma. The presented associations between vitamin D signaling elements and uveal melanoma in comparison to uveal tract encourage future clinical research with larger patients’ population.
Subject terms: Eye cancer, Predictive markers, Prognostic markers, Outcomes research, Eye cancer
Introduction
Melanomas developing from ocular tissues make up to 5% of all melanomas. Among them, 5% are conjunctival melanomas, 85% uveal melanomas, and 10% are lesions occurring in other ocular structures1.
Uveal melanoma is the most frequent primary intraocular tumor in adults. The most frequent location in uveal melanomas is the choroid (90%), less frequent – the ciliary body (6%), while the iris is the least frequent location (4%)2. The prevalence varies depending on the population studied: in the USA, this is 5.1 new cases per 1 million inhabitants per year3,4. In Europe, the prevalence of this tumor is determined by latitude: in the countries of Southern Europe (Italy, Spain) these are 2 cases per 1 million per year, whilst in the Northern part of the continent (Norway, Denmark) – 84,5. In Asia and Africa, the prevalence is assessed to be about 0.2 to 0.3 new cases per 1 million per year3,6. This type of cancer is the most frequent among Caucasians (98%)3,7. Uveal melanoma may occur at any age, yet it is quite rare among children and young adults8. The incidence increases with age, reaching a constant level at the age of 75, whereas the average age when this cancer is diagnosed ranges from 59 to 622–4.
Thanks to the current methods of uveal melanoma treatment, the rate of positive effects of local treatment is about 76–100%9,10. Unfortunately, there are still no effective treatment methods which would allow to cure the disease completely or to prolong significantly the survival of the patients in whose case tumor spread occurred. An average survival period from the moment of diagnosing distant metastases is 4–15 months, while only 10–15% patients survive 1-year11,12. The 5-year mortality in patients with uveal melanoma is 31%, while the 15-year mortality is 45%13. This brings the necessity of a farther search for new mechanisms which affect the oncogenesis of this tumor, its metastases and also for the prognostic markers allowing better assessment of disease outcome and facilitating the selection of the proper treatment and follow-up pattern.
Within the last few years, the number of studies concerning the function of vitamin D and its effect on human disease has significantly increased. Vitamin D is a prohormone that is produced in the skin through photochemical transformation of 7-dehydrocholesterol upon exposure to ultraviolet radiation B (UVB)14–19. Vitamin D is activated through sequential hydroxylations at C25 (by CYP27A1 and/or CYP2R) with following hydroxylation at C12 by CYP27B1 and inactivated by CYP24A1 through hydroxylation at C24 with further shortening at the side chain to produce calcitroic acid14–17. Via its interaction with the vitamin D receptor (VDR) active form of vitamin D3, 1,25-dihidroxyvitaminD3 (1,25(OH)2D3) regulates the transcription of many genes16,17,20. In addition to its role in the regulation of calcium and phosphate metabolism, vitamin D has many other functions that include the regulation of inflammation and immune functions (inhibiting autoimmune processes), stimulation of cell differentiation and maturation, inhibition of cell proliferation, regulation of endocrine functions (e.g., insulin secretion). In addition, It has been reported to stimulate neuron regeneration, increase muscle mass and strength, stimulate hepatic cell regeneration, inhibit the development of depression and schizophrenia, affect the circulatory system through renin-angiotensin-aldosterone system, and decrease the risk of oral decay21–23.
Another important functions of active form of vitamin D are its anti-tumor effects, antimutagenic properties (e.g. protection against the effect of free radicals) and the regulation of tumor proliferation, apoptosis, and angiogenesis24,25.
It was shown that vitamin D deficiency or dysregulated vitamin D signaling can play an important role in oncogenesis, clinical advancement and prognosis in such neoplasms as cutaneous melanomas, bladder, breast, lung, ovarian, pancreatic, thyroid, prostate and colorectal cancers26–36. In addition, amounts in expression of its receptor (VDR), expression of hydroxylases participating in its activation and metabolism (CYP27B1, CYP24A1), as well as the expression of the alternative receptors, retinoic acid-related orphan receptors α and γ (RORα and RORγ) in tumor tissues can affect the outcome of the disease26–29,31–37.
Because of such versatile actions of vitamin D, more and more scientists have taken up the research concerning its function in ophthalmologic diseases. Decreased levels of vitamin D metabolites in blood serum have been linked to an increased risk of age-related macular degeneration (AMD), dry eye syndrome, diabetic retinopathy and impaired corneal healing after surgeries and traumas38–42.
The studies carried out by Alsalem et al. confirmed the presence of VDR and CYP27A1, CYP2R1, CYP27B1, CYP24A1 in the cells of the protective barrier of human eye, i.e. primary human scleral fibroblasts (HSF), in cultured corneal endothelial cells (HCEC-12), adult retinal pigment epithelial (ARPE-19), and nonpigmented ciliary body epithelial cells (ODM-2)43.
Other receptors linked to the control of cellular functions by sterols44–46, including vitamin D47,48 and lumisterol compounds49, are the nuclear receptors RORα and RORγ. These transcription factors regulate several physiological and pathological processes, including various immune and endocrine functions, as well as cell growth, differentiation, apoptosis, and cancer50–56. RORα and RORγ also participate in the regulation of circadian rhythms by directly regulating the expression of a number of clock genes57,58.
New vitamin D hydroxyderivatives that can also act as inverse agonists on ROR are produced ex-vivo by adrenal and placenta fragments, epidermal keratinocytes and are detectable in human epidermis and serum59–61. It has been recently demonstrated that RORα and RORγ are expressed in normal and pathological human skin and that hydroxyderivatives of vitamin D can act as inverse agonists of ROR47,49,54. In cutaneous melanoma a inverse correlation between the RORα and RORγ expression and the progression of human cutaneous melanoma has been observed62.
To fill the gap in our knowledge on vitamin D activity in uveal melanoma, we decided to evaluate the expression pattern of the VDR, CYP27B1 and CYP24A1 hydroxylases and RORα and RORγ in the cells of uveal melanoma, in normal melanocytes and in other normal uveal cells in humans. Additionally, the relationship between VDR, CYP27B1, CYP24A1, RORα and RORγ expression, with clinical presentation and melanin content were examined.
Results
Expression of the VDR in human uveal tract and uveal melanoma
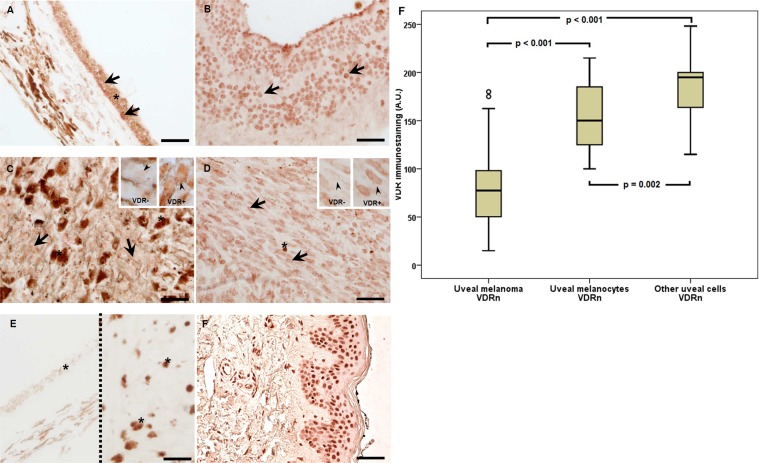
Similarly as in the cells of cutaneous melanoma, VDR immunostaining has been found in uveal melanoma both in cell nuclei (VDRn) and in cytoplasm (VDRc). The presence of nuclear vitamin D receptor (VDRn) was observed in all types of the uveal tissues of adult patients. VDRn was present both in the tumor cells (uveal melanoma, 100%), and in non-tumor cells, such as normal melanocytes (uveal melanocytes, 96.77%) and other normal cells of the surrounding connective tissue (fibroblasts, 70.97%) (Fig. 1A–E). The difference in the number of evaluated receptors between normal and tumor tissue was statistically significant. In the tumor cells, the level of VDRn was significantly lower (the Wilcoxon signed-rank test, p < 0.001). The analysis showed also a statistically significant difference between the numbers of VDRn in normal cells of the uveal tissue. Melanocytes contained less VDRn than other cells of connective tissue did (the Wilcoxon signed-rank test, p = 0.002) (Fig. 1F). In uveal melanoma, the presence of cytoplasmic vitamin D receptors (VDRc) was found (Me = 25.0, and Q1 = 10, Q3 = 43.75).
Figure 1.
Representative VDR immunostaining in uveal pigmented cells (A), other cells (B), strongly pigmented (C) and slightly pigmented (D) melanomas and negative (E; two separate samples separated with dotted line) and positive (F) controls. Inserts present high magnification of negative (VDR−) and positive (VDR+) cell nuclei (as indicated by arrow heads). Arrows indicate nuclear VDR immunostaining, asterisks indicate melanin, scale bar: 50 μm. Mean nuclear VDR immunolabelling (A.U. – arbitrary units) in melanoma, normal melanocytes and other normal uveal cells (F) (statistically significant differences are denoted with the Wilcoxon signed-rank test).
CYP24A1 and CYP27B1 in human uveal tract and uveal melanoma
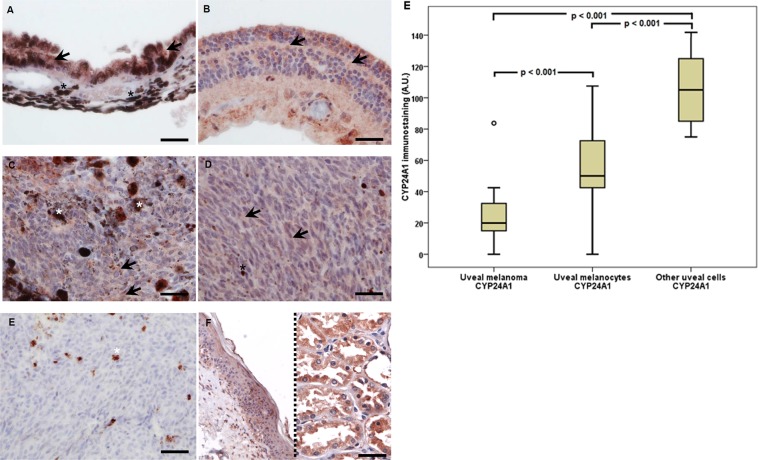
CYP24A1, a mitochondrial monooxygenase belonging to the cytochrome P450 – superfamily, was found to be expressed in melanoma cells (90.32%), normal uveal melanocytes (67.74%) and other normal cells (54.84%) of the choroid coat (Fig. 2A–E). Mitochondrial monooxygenase is an enzyme, whose core function is the hydroxylation of an active form of 1.25-dihydroxyvitamin D3 into its inactive one. The lowest average values of this enzyme were found in the melanoma cells, larger ones – in normal melanocytes, whilst the largest – in other normal uveal cells.
Figure 2.
Representative CYP24A1 immunostaining in uveal pigmented cells (A), other cells (B), strongly pigmented (C) and slightly pigmented (D) melanomas and negative (E) and positive (F) controls. For CYP24A1 in addition to normal skin (left) also kidney (right) samples served as positive controls. Arrows indicate CYP24A1 immunostaining, asterisks indicate melanin, scale bar: 50 μm. Mean CYP24A1 immunolabelling in melanoma, normal melanocytes and other normal uveal cells (F) (statistically significant differences are denoted with the Wilcoxon signed-rank test).
The Wilcoxon signed-rank test showed that the immunostaining CYP24A1 distribution in uveal melanoma and uveal melanocytes, uveal melanoma and other uveal cells significantly differ from each other (p < 0.001) (Fig. 2F).
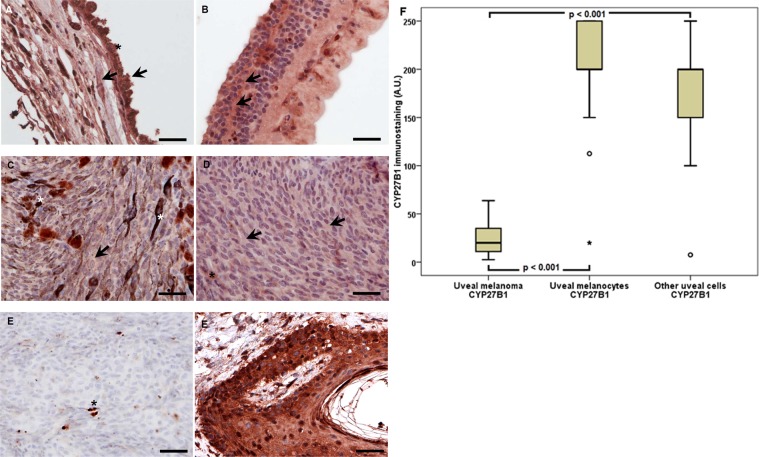
The presence of another enzyme belonging to cytochrome P450 superfamily, CYP27B1, and also known as 1-alpha-hydroxylase was found in 96.77% melanoma, 100% melanocytes, and in 70.97% other normal cells of the choroid coat (Fig. 3A–E). This enzyme catalyzes calcidiol (25(OH)D3) hydroxylation to calcitriol, i.e. 1,25(OH)2D3, the bioactive form of vitamin D.
Figure 3.
Representative CYP27B1 immunostaining in uveal pigmented cells (A), other cells (B), strongly pigmented (C) and slightly pigmented (D) melanomas and negative (E) and positive (F) controls. Arrows indicate CYP27B1 immunostaining, asterisks indicate melanin, scale bar: 50 μm. Mean CYP27B1 immunolabelling in melanoma, normal melanocytes and other normal uveal cells (F) (statistically significant differences are denoted with the Wilcoxon signed-rank test).
In uveal melanoma, the values of CYP27B1 labelling were significantly lower than in normal melanocytes and other uveal cells (the Wilcoxon signed-rank test, p < 0.001) (Fig. 3F).
RORα and RORγ in human uveal tract and uveal melanoma
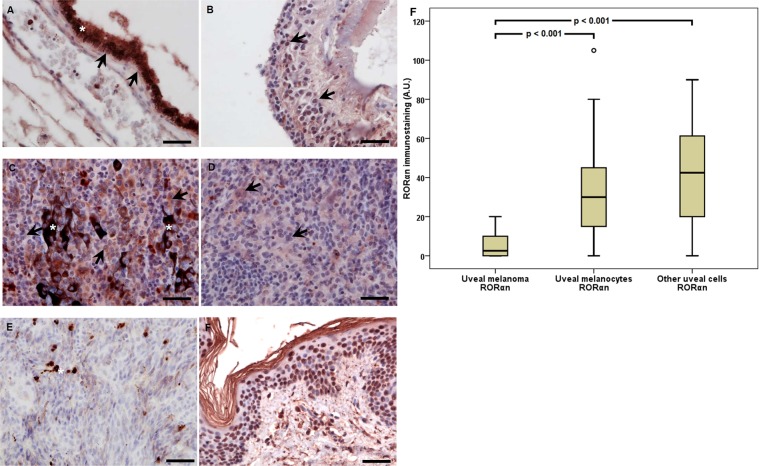
Similarly, to cutaneous melanoma cells, RORα and RORγ were found to be expressed in both the nuclei (RORαn, RORγn), and the cytoplasm (RORαc, RORγc) of uveal melanoma and normal tissue. RORα and γ (NR1F1 and NR1F3, respectively) are member of the nuclear receptor superfamily47 that regulate gene transcription in many cell types. In our study, we observed expression of RORα in the nuclei (RORαn) of melanoma cells (93.55%), normal uveal melanocytes, (54.84%) and other normal uveal cells (64.52%) (Fig. 4A–E). The values of immunostaining of RORαn differed significantly between the tumor cells of the uveal melanoma, normal melanocytes and other uveal cells (the Wilcoxon signed-rank test, p < 0.001). Concerning differences between normal melanocytes and other normal uveal cells, there were no differences in immunostaining (the Wilcoxon signed-rank test, p = 0.557) (Fig. 4F). RORα immunostaining of the cytoplasmic component in the uveal melanoma cells was found in 93.55% of cells. Median was 2.50, and Q1 = 0.00 and Q3 = 7.50.
Figure 4.
Representative RORα immunostaining in uveal pigmented cells (A), other cells (B), strongly pigmented (C) and slightly pigmented (D) melanomas and negative (E) and positive (F) controls. Arrows indicate RORα immunostaining, asterisks indicate melanin, scale bar: 50 μm. Mean RORα nuclear (RORαn) immunolabeling in melanoma, normal melanocytes, and other normal uveal cells (F) (statistically significant differences are denoted with the Wilcoxon signed-rank test).
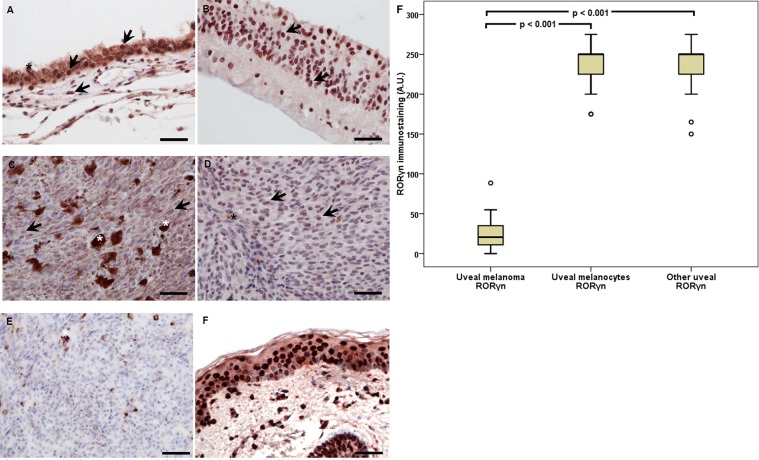
RORγ expression was observed in 93.55% of melanoma cells and in normal melanocytes (93.55%), as well as in other normal cells (77.40%) (Fig. 5A–E). Similar to RORα, the lowest level of nuclear RORγ was observed in the uveal melanoma cells. Nuclear immunostaining (median value) of this receptor differed between the tumor cells of the uveal melanoma and normal melanocytes and other normal uveal cells (the Wilcoxon signed-rank test, p < 0.001), with the lack of statistical significance between normal melanocytes and other normal uveal cells (the Wilcoxon signed-rank test, p = 0.718) (Fig. 5F). In uveal melanoma cells, also RORγc cytoplasmic immunostaining (93.55%) was observed. The median was 10.00, and Q1 = 5.00 and Q3 = 17.50.
Figure 5.
Representative RORγ immunostaining in uveal pigmented cells (A), other cells (B), strongly pigmented (C) and slightly pigmented (D) melanomas and negative (E) and positive (F) controls. Arrows indicate RORγ immunostaining, asterisks indicate melanin, scale bar: 50 μm. Mean RORγ nuclear (RORγn) immunolabelling in uveal melanoma, normal melanocytes and other normal uveal cells (F) (statistically significant differences are denoted with the Wilcoxon signed-rank test).
The correlation of VDR immunostaining and melanin in uveal melanoma
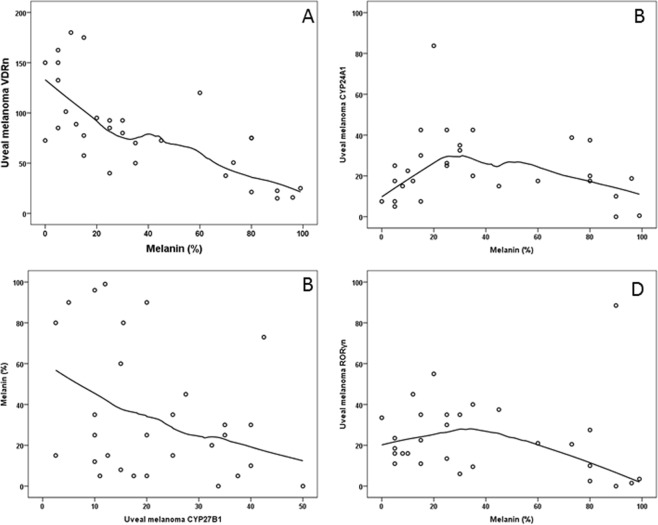
The analysis of VDRn, VDRc and all VDR (VDRa = VDRn + VDRc) immunostaining and the degree of melanin pigmentation in uveal melanoma cells showed a negative correlation with the levels of VDRn and VDRa to melanin (Spearman’s rank correlation r = −0.704, p = 0.001 and r = −0.666; p = 0.004 respectively) (Fig. 6A). No such significant correlation was found for VDRc (r = −0.320, p = 0.079).
Figure 6.
The correlation of: VDRn immunostaining and the level of melanin (A); CYP24A1 immunostaining and melanin (B); CYP27B1immunostaining and melanin (C); RORγn immunostaining and melanin (D).
Correlation between CYP24A1 and CYP27B1 immunostaining and melanin content in uveal melanoma
The correlation was statistically significant only for CYP24A1 expression in comparison to the melanin levels in uveal melanoma (p = 0.020). With low melanin levels, the level of CYP24A1 was the lowest (mean level = 14.6875, SD = 7.37243), reaching the highest values at medium melanin level (mean level = 33.5417, SD = 719.32698), and reversed with the highest levels of melanin (mean level = 17.8333, SD = 13.73806) (Fig. 6B).
No significant correlations between CYP27B1 labelling and melanin were found. The graph shows that CYP27B1 level has the trend to decrease when the melanin content increases, yet this is not statistically significant (rho = −0.30, p = 0.111) (Fig. 6C).
Correlation between RORα and RORγ immunostaining and melanin content in uveal melanoma
The evaluation of RORα and melanin level in uveal melanoma did not reveal any significant correlations. Solely a tendency to reverse proportion (p = 0.576) was observed. For lower melanin values, the level of RORαn was increased.
The analysis of the correlation between RORγ immunostaining and melanin in the tumor cells showed statistical correlation, indicating that RORγn was higher in the tumors with a lower (<50%) melanin level (p = 0.030) (Fig. 6D).
The relationship between VDRn, VDRc, VDRa and CYP24A1 and CYP27B1 in uveal melanoma
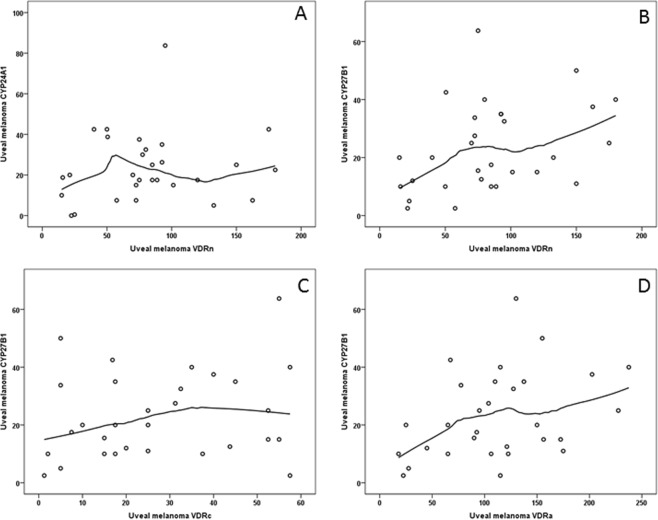
No significant correlations between VDR and CYP24A1 in the cells of uveal melanoma were found. However, there was a tendency observed, indicating that together with an increase of VDRn to the level of about 40, the CYP24A1 value also increases (Fig. 7A).
Figure 7.
The correlation between VDRn immunostaining and CYP24A1 level (A). The correlation of the level of CYP27B1 to VDRn (B), VDRc (C) and VDRa (D).
The levels of VDRn and VDRa increased together with those of the CYP27B1 value (Spearman’s rank correlation, rho = 0.401, p = 0.028 and rho = 0.390, p = 0.033, respectively) in uveal melanoma cells. For VDRc a tendency of reverse correlation is observed, but without a statistical significance p = 0.254 (rho = 0.22) (VDRn, VDR and VDRa to CYP27B1) (Fig. 7B–D).
Correlation between VDR immunolabeling and RORα and RORγ in uveal melanoma
The analysis of VDR and ROR immunostaining in the tumor cells revealed a statistically significant positive correlation between RORαn and VDRn in the melanoma cells (p = 0.030). No such correlation in the melanoma cells was found between RORαn and VDRc and VDRa (p = 0.476 and p = 0.118 respectively). In the melanoma cells, increased levels of RORγn correlated with increased levels of VDRn (p = 0.006) and VDRa (p = 0.022). No correlation was found between the levels of RORα or RORγ and CYP24 or CYP27.
The relationship between VDR and clinical and pathomorphological features
Lack of statistically significant correlations were found between the levels of VDR (with one exception described above), CYP27B1, CYP24A1 and ROR, and histopathological diagnosis of uveal melanoma (spindle-cell melanoma, mixed and epithelioid cell-type), the degree of sclera infiltration, the degree of optic disc infiltration, the presence of tumor cell emboli in the scleral vessels, the involvement of the ciliary body, tumor size (the largest base diameter and height), tumor shape (mushroom-shaped, dome-shaped and diffuse), the clinical advancement stage (AJCC) and 2-year survival. Some tendencies could be observed, yet without statistical significance.
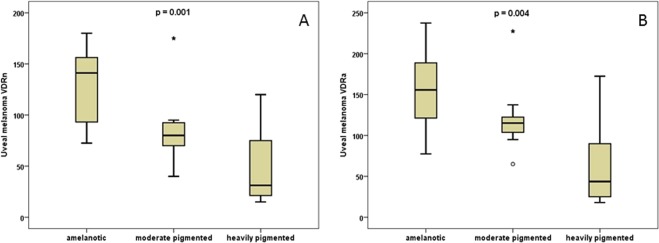
The only exception was the statistically significant correlation between the levels of VDRn and VDRa, and clinically assessed degree of tumor pigmentation (p = 0.001 and p = 0.004 respectively). In amelanotic tumors, the levels of VDRn were the highest, then VDRn decreased in medium-pigmented ones, whilst the lowest values were observed in the strongly-pigmented (dark brown) uveal melanomas (Fig. 8A). The same correlation was found for VDRa (Fig. 8B). No such correlation was found for VDRc (p = 0.072).
Figure 8.
Comparison of VDRn (A) and VDRa (B) expression with the clinical assessment of tumor pigmentation (the Wilcoxon signed-rank test).
Discussion
In this study, we provide evidence for the existence of vitamin D signaling in normal uveal cells (fibroblasts and melanocytes) and in tumors originating from this part of the eyeball in humans, i.e. uveal melanoma. Our study provides evidence showing the presence of VDR and CYB27B1 and CYP24A1, hydroxylases taking part in vitamin D metabolism, as well as RORα and RORγ, alternative receptors for vitamin D-hydroxyderivatives.
In all of the tissues examined the presence of nuclear receptors for vitamin D (VDRn), RORαn and RORγn, as well as CYB27B1 and CYP24A1, were confirmed. Moreover, cytoplasmic expression of VDR, RORα and RORγ (VDRc, RORαc and RORγc) was observed in the cells of uveal melanoma. The levels of VDR, CYP24A1, CYP27B1 and RORs were always the lowest in the cells of uveal melanoma and higher in normal uveal melanocytes and other normal uveal cells.
The remaining challenge is to establish molecular principles underlying the differences of protein expression between malignant and non-malignant samples. Since current study are focused on the paraffin embedded archival tissues, future studies on samples collected from the patients would clarify at which level these events are regulated, e.g., gene transcription, RNA processing, translation or posttranslational modifications.
With respect to other tumors, a similar decrease in the level of the above markers was observed in comparison to normal cells surrounding the tumor. In the case of breast cancer, Al.-Azhri et al. observed reduced VDR expression that was inversely correlated with the aggressive tumor characteristics, including large tumor size, hormonal receptor (HR) negativity, and triple-negative subtype (P < 0.05)63,64.
In ovarian cancers, normal ovarian epithelium showed the highest levels CYP27B1, opposed to a significant decrease of its expression in cancer tissue31. In urinary bladder cancer, the level of VDR and CYP27B1 in tumor tissues was significantly lower than in normal epithelium, which was observed by Jóźwicki et al.29. Bises and colleagues found a higher CYP27B1 expression in well-differentiated colon cancers compared with normal mucosa; however, in tumor areas showing a considerably poor differentiation, this expression was lost56. Also in skin melanoma, a significantly decrease in the expression of VDR, CYB27B1, CYP24A1, RORα and RORγ was observed in comparison to normal melanocytes or perilesional keratinocytes28,62,65–67.
In the current study a reverse correlation was found between the melanin level and VDR in uveal melanoma. The same correlation was observed when the degree of lesion pigmentation was analyzed in the clinical picture in comparison with the nuclear VDR. In predominantly amelanotic tumors, the levels of VDR were the highest, then VDRn decreased in medium-pigmented (dark brown) uveal melanomas. Similar observations were made in the studies cutaneous melanoma, where the level of VDR decreased together with an increase of melanin content and poorer prognosis27,65. It was also shown that melanogenesis induction in human SKMel-188 melanoma cells was accompanied by a marked decrease in VDR expression and responsiveness to active forms of vitamin D26,68. Several reports have indicated that in uveal melanoma, the risk of metastases increases with increased tumor pigmentation7,69. In the above context, the loss of the protective function of vitamin D could be secondary to the reduction of the VDR expression by an increased melanogenesis. Of note, melanogenesis can affect biological functions of melanoma cells70–75 and normal melanocytes76,77.
Vitamin D metabolism is connected to the CYPs expression, which catalyze the reaction in which the active form of vitamin D is formed or metabolized to biologically inactive forms. In our study we confirmed the presence of CYP27B1 and CYP24A1 in the melanoma cells and their connection with the VDR level. Together with an increase of the CYP27B1 value, the levels of VDRn and VDRa were increasing and, for VDRc, a tendency of a direct proportionality was observed. In the case of CYP24A1, together with an increase of VDRn in the melanoma to the level of about 40, the CYP24A1 value also increased. However, once this value was reached, there was a lack of statistical significance for any further correlation. The above results are similar to those reported for other tumors including cutaneous melanoma66.
Thus, the decreases in both CYP27B1 and VDR expression in uveal melanomas could reflect defects in the local vitamin D signaling pattern, which could facilitate melanoma progression and invasion. Similar findings were made by Brożyna et al. in a study on cutaneous melanomas67. Patients with low CYP27B1 and VDR expression had a shorter overall survival. Moreover, an absence of CYP27B1 or a reduction promoted the development of metastases and correlated with shorter disease-free survival28,65,67. A correlation was found between the level of CYP24A1 and melanin in uveal melanoma, yet no correlations were observed between the CYP27B1 labelling and melanin.
With respect to ROR expression in uveal melanomas, it was found that together with an increase of RORαn, the level VDRn increased significantly and, together with an increase of RORγn in melanoma, the levels of VDRn and VDRa (p = 0.022) also increased. No significant correlations between RORαn and VDRc or VDR and between RORγn and VDRc were found, yet some tendency of direct proportional correlation was observed in these cases.
Our analysis on the correlation between RORα and RORγ and the presence of melanin in uveal melanoma, showed only a tendency for reverse correlation for RORα. Thus, lower levels of melanin was associated with increased expression of RORαn. Likewise, RORγn was higher in the tumors with a lower level of melanin. These observation are in agreement with observations made in cutaneous melanomas the expression level of RORα and RORγ decreased in strongly pigmented melanomas62.
Vitamin D has the number of confirmed anti-tumor functions, acting through VDR, such as inhibition of cell proliferation, accelerated apoptosis, regulation of the cell cycle and differentiation, among others thanks to the presence of FOXO protein, cyclin-dependent kinase inhibitors like p21 or p27, insulin-like growth factor (IGF), transforming growth factor beta (TGFβ), and Wnt-β/catenin24. These also include anti-tumor effects in cutaneous melanomas26,27. Therefore, we propose that higher levels of VDR in uveal melanoma might be connected with a better prognosis in this disease. Taking into consideration that metastases of uveal melanoma, as opposed to cutaneous melanoma, can occur after many years, further observation of the reported patients during the follow-up period is required for definitive conclusions. The confirmation of the above pattern on a larger group of patients with simultaneous correlation with the clinical outcome are required to better define the VDR as a prognostic factor. This would open the way for creating new adjuvant therapies of uveal melanoma that would base on vitamin D derivatives, as it has been demonstrated for other tumors including cutaneous melanoma. Additional open questions are whether the levels of VDR, CYP27B1 and CYP24A1 and ROR in the tumor cells can also serve as reliable prognostic factors and in what way the level of VDR may affect the efficiency of vitamin D in inhibiting the tumor growth and aggressiveness.
In conclusion, we provide an evidence for the presence of the VDR, CYB27B1, CYP24A1, RORα and RORγ in uveal cells, which changes in uveal melanomas and suggest that these proteins are involved in clinical presentation and represent targets for novel therapeutic approaches.
Materials and Methods
The study involved 31 adult patients in whom, on account of uveal melanoma, the eyeballs were enucleated during the period between 2013 and 2014 in the Department of Ophthalmology and Ocular Oncology, Medical College of Jagiellonian University in Kraków, Poland. For all patients, enucleation was the only way to treat the affected eye, with no previous local or systemic therapies. The study group comprised 19 women (61.30%) and 12 (38.70%) man, at the age ranging from 39 and 83 years (mean age: 62.9, SD = 10.8). The tissues of the enucleated eyeball were examined, with special attention paid to the medium layer, i.e. the choroid coat and to the primary tumor – melanoma originating from pigment cells (melanocytes) of this part of the eye. The disease affected the left eye more frequently (22 cases; 71.00%) than the right one (9 cases; 29.00%). The clinical TNM stage of the cancer was T3 in 17 cases (54.80%) cases and T4 in 14 cases (45.20%), whilst the AJCC stage was in 4 cases (12.90%) IIa, and in 13 cases – (41.90%) IIb, in 7 cases (22.60%) – IIIa, in 4 cases (12.90%) IIIb and in 3 cases (9,70%) IIIc78.
In the ultrasound picture, a mushroom-shaped tumor was dominating (19; 61.30%), followed by dome-shaped one (11; 35.50%), and only in 1 case (3.20%) a diffuse shape was observed. In all cases, histopathological assessment confirmed the diagnosis of uveal melanoma. In 25 cases (80.60%) it was melanoma of the choroid coat, in 4 cases (12.90%) of the choroid coat and ciliary body, and in 2 cases (6.50%) of the choroid coat, ciliary body and iris. On the basis of the modified Callender’s classification of uveal melanoma, the following histopathological subtypes of melanoma were diagnosed: spindle-cell – in 11 cases (35.50%), mixed – in 13 cases (41.90%) and epithelioid cell-type in 7 cases (22.60%). The eyeball infiltration and extra-bulbar infiltration were observed in 6 cases (19.40%) and in 5 cases (16.10%) cases respectively. Necrosis was found in 3 (9.75%) tumors. Optic disc involvement was observed in 11 cases (35.50%), and the involvement of ciliary body in 6 (19.40%) tumors.
The degree of tumor pigmentation (light, i.e. predominantly-amelanotic, medium-pigmented and strongly-pigmented, i.e. dark-brown) was assessed by 2 ophthalmologists working independently, during the enucleation procedure (after the resection, in order to collect the study specimen) and/or during an ophthalmoscopic assessment or on the basis of the evaluation of the photograph of the patient eye fundus. Additionally, for the purpose of further analysis, the percentage content of melanin in all the histopathological specimens was made, distinguishing 3 groups depending on the melanin level: 0–10 (predominantly-amelanotic), 11–50 (medium-pigmented) and 51–100 (with strong pigmentation). In all the cases, the clinical assessment and histopathological assessment overlapped. In the analyzed tumors, 8 predominantly-amelanotic types (25.80%), 13 medium-pigmented types (41.90%) and 10 types with strong pigmentation (32.30%) were found.
The follow-up period was 2 years. The analysis of the overall survival (OS) in this period found that 8 patients (25.80%) died during that period, out of whom 6 patients (19.40%) died of the dissemination of uveal melanoma, 1 person (3.20%) died of a different cancer and another 1 person (3.20%) died of a sudden cardiac arrest. In the evaluation of the disease free survival (DFS) in that period, no case of local recurrence in the orbit was found, but in 8 patients (25.80%), cancer dissemination was found on additional assessments. In all these cases, some numerous isolated metastases in the liver were found and, additionally, in 1 case (3.20%) melanoma foci were observed in the lungs.
In all the specimens, the tissue of uveal melanoma, normal melanocytes and connected tissue cells of the choroid coat were analyzed with regards to the presence of nuclear and cytoplasmic vitamin D receptors for and enzymes taking part in its metabolism, such as CYP27B1 and CYP24A1, in the manner described below. In these cells also the presence of RORα and RORγ was assessed.
After the presence of all the above markers was found, their qualitative and quantitative analyses were made and then, with the use of statistical analysis tools, the correlations between these markers and also between these markers and the clinical features (including pathomorphological ones) of uveal melanoma were evaluated.
Authors declare that this investigation was carried out following the rules of the Declaration of Helsinki of 1975 (revised in 2008) and this study was approved by the Ethics Committee of the Jagiellonian University, Krakow, Poland (No 1072.6120.34.2017, 20 June 2017). The Bioethical Committee of the Jagiellonian University (Poland), which gave their permission for conducting this study, waived the requirement to obtain Patients’ informed consent for this research.
VDR, CYP27B1, CYP24A1, RORα and RORγ immunohistochemistry
VDR, CYP27B1, CYP24A1, RORα and RORγ expression was detected using immunohistochemistry as previously described28,29,31,61,65,66,79 with slight modifications. Briefly, formalin-fixed, paraffin-embedded 4µm-sections, after antigen retrieval in high pH buffer using PT-Link device (Dako, Glostrup, Denmark), were stained overnight using appropriate antibodies (Table 1). Imm-PACT NovaRED (Vector Laboratories Inc., Burlingame, CA, USA) was used as a substrate for peroxidase for visualization. Then, sections were counterstained with hematoxylin (except VDR immunostaining), and mounted under the non-aqueous medium (Consul Mount; Thermo Fisher Scientific Inc. Waltham, MA, USA). For negative controls primary antibodies were replaced with antibody diluent. Samples of normal skin served as positive control. In addition, for CYP24A1 kidney samples were used as additional positive controls.
Table 1.
Description of the antibodies used and their manufacturers, and of immunocytochemistry protocols and detection methods.
| Antibody | Vendor | Permeabilization/blocking before primary antibodies application | Primary antibody (dilution; incubation time) | Secondary antibody (dilution; incubation time; vendor) |
|---|---|---|---|---|
| Anti-VDR, rat monoclonal, clone 9A7 | Abcam (Abcam Inc., Cambridge, MA, USA) | NA | 1:50, O/N, 4 °C | anti-rat, peroxidase-conjugated goat antibody (1:250, 30 min, Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, USA); |
| Anti-CYP27B1, rabbit polyclonal, clone H-90 | Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) | 0.2% Triton-X100, 15 min, RT | 1:75, O/N, 4 °C | EnVision System-HRP (RTU, 30 min, antibody with peroxidase-conjugated polymer backbone,Dako, Carpinteria, CA, USA) |
| Anti-CYP24A1, mouse monoclonal, | Abcam (Abcam Inc., Cambridge, MA, USA) | 0.2% Triton-X100 in 1% BSA, 25 min, RT | 1:30, O/N, 4 °C | EnVision System-HRP (RTU, 30 min, antibody with peroxidase-conjugated polymer backbone, Dako, Carpinteria, CA, USA) |
| Anti-RORα, goat polyclonal, clone C-16 | Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) | 2% BSA, 20 min, RT | 1:500, O/N, 4 °C | EnVision System-HRP (RTU, 30 min, antibody with peroxidase-conjugated polymer backbone,Dako, Carpinteria, CA, USA) |
| Anti-RORγ, rabbit | customer-made at NIEHS by Dr Jetten* | 0.1% Triton-X100 in 3.5% BSA, 60 min, RT | 1:50, O/N, 4 °C | EnVision System-HRP (RTU, 30 min, antibody with peroxidase-conjugated polymer backbone,Dako, Carpinteria, CA, USA) |
Evaluation of immunostained sections has been performed by two independent observers in a blind manner, without knowing the detailed histopathological diagnosis and the clinical data. VDR, CYP27B1, CYP24A1, RORα and RORγ immunostaining of melanomas was scored semiquantitatively as previously described and both staining intensity, using a four-point scale, and percentage of cells was evaluated and calculated as follows: SQ = mean(IR × SI)/100, where IR is the percentage of immunoreactive cells and SI is the staining intensity (assessed using the scale from 0 to 3 arbitrary units (A.U.). The staining intensity in positive controls served as reference for staining intensity evaluation in samples of uveal melanoma samples. Since the immunostaining of CYP24A1 in normal skin samples were heterogenous between different samples, CYP24A1 were assessed in relation to its expression in the kidney28,29,31,61,65,66,79.
Statistical analysis
The distribution of qualitative variables was described with the absolute numbers in specific categories (n) and their percentage distribution in that variable (%). Average values of variables with normal distribution were described with the mean value and standard deviation (SD), whilst in the case of variables whose distributions were significantly different from the normal ones – with the median and quartiles: the first and the third ones. The compliance of the distribution of a given variable with the normal distribution was assessed with the Shapiro-Wilk test, and with skewness and also it was visually evaluated by means of histogram analysis, boxplot and Q-Q plot (quantile-quantile).
Distributions of the studied variables were presented using boxplots, where bolded line at the interior of the box represents median, lower and upper end of the box (hinges) represent the 1st and the 3rd quartile, respectively. Whiskers represent observations ranging up to 1.5 of interquartile range from the box, circles represent observations ranging from 1.5 up to 3 interquartile ranges above or below from the hinges, and asterisks represent observations lying above or below 3 interquartile ranges from the hinges.
Relationships between particular variables were presented as scatterplots with added locally smoothed lowest curve, estimated using Epanechnikov algorithm.
The comparison of mean values of the variables with normal distribution in two nonrelated groups was made with the t-Student test for independent groups, whilst in more than two unrelated groups – with the ANOVA analysis of variance. The comparison of mean values of variables with distribution different significantly from normal ones in two unrelated groups was made with the Mann-Whitney test, whilst in more than two unrelated groups with the Kruskal-Wallis test. The comparison of mean values of the variables with the distributions different significantly from normal ones in two unrelated groups was made with the Wilcoxon test for related variables measured on interval or ordinal scale.
The strength of the correlation between the variables measured in interval scale was evaluated with the Pearson correlation coefficient, when between the variables there was a straight-line relationship, whilst, with Spearman’s Rho coefficient when the relationship was monotonous, but not a straight-line one, and in all other cases – with the eta correlation coefficient. A graphic presentation of the correlations between the analyzed variables was made with a scatterplot with the plugged loess curve, illustrating mean values of the variable presented on the Y axis and corresponding to the values of the variable presented in the X axis. The effects with the value p < 0.05 were regarded as statistically significant. The calculations were made with the IBM SPSS Statistics 24 for Windows software.
Acknowledgements
This study was supported partially by grant: 2012/07/B/NZ4/01657 from Polish National Science Centre to KU. This study was supported in part by grant 2014/15/B/NZ4/00751 from National Science Centre, Poland to AAB. Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a partner of the Leading National Research Center (KNOW) supported by the Ministry of Science and Higher Education. Partial support from NIH grants 1R01AR071189-01A1 and R01AR073004 and VA merit grant (no. 1I01BX004293-01A1) to ATS is also acknowledged. AMJ research was supported by the Intramural Research Program of the NIEHS, NIH Z01-ES-101586. Partial support to ME was given by Horizon2020 grant UMCure2020 no 667787.
Author Contributions
Anna Markiewicz prepared text of the publication, described the clinical aspects, performed literature search and statistical analysis, Anna A. Brożyna performed immunohistochemistry and contributed to writing of the manuscript, Ewa Podgórska performed immunohistochemistry, Martyna Elas managed the references, corrected the text, and performed literature research, Anton M. Jetten prepared antibodies for RORs and contributed to writing of the manuscript, Krystyna Urbańska, Andrzej T. Słominski and Bożena Romanowska-Dixon delineated the concept of the study and contributed to writing of the manuscript, Wojciech Jóźwicki outlined concept of the paper, Jolanta Orłowska-Heitzman and Grzegorz Dyduch prepared histopathological materials.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base Report on Cutaneous and Noncutaneous Melanoma. Cancer. 1998;83:1664–1678. doi: 10.1002/(SICI)1097-0142(19981015)83:83.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009;127:989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 3.Kaliki S, Shields CL. Uveal melanoma: Relatively rare but deadly cancer. Eye. 2017;31:241–257. doi: 10.1038/eye.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh AD, Turell ME, Topham AK. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 5.Virgili Gianni, Gatta Gemma, Ciccolallo Laura, Capocaccia Riccardo, Biggeri Annibale, Crocetti Emanuele, Lutz Jean-Michel, Paci Eugenio. Incidence of Uveal Melanoma in Europe. Ophthalmology. 2007;114(12):2309-2315.e2. doi: 10.1016/j.ophtha.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Kivelä T. The epidemiological challenge of the most frequent eye cancer: Retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93:1129–1131. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 7.Shields CL, et al. Prognosis of uveal melanoma based on race in 8100 patients: The 2015 Doyne Lecture. Eye. 2015;29:1027–1035. doi: 10.1038/eye.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Jamal RT, et al. The pediatric choroidal and ciliary body melanoma study: A survey by the European Ophthalmic Oncology Group. Ophthalmology. 2016;123:898–907. doi: 10.1016/j.ophtha.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Echegaray JJ, Bechrakis NE, Singh N, Bellerive C, Singh AD. Iodine-125 Brachytherapy for Uveal Melanoma: A Systematic Review of Radiation Dose. Ocul. Oncol. Pathol. 2017;3:193–198. doi: 10.1159/000455872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma V, Mehta MP. Clinical Outcomes of Proton Radiotherapy for Uveal Melanoma. Clin Oncol. 2016;28:17–27. doi: 10.1016/j.clon.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Diener-West M, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123:1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 12.Postow MA, Kuk D, Bogatch K, Carvajal RD. Assessment of overall survival from time of metastastasis in mucosal, uveal, and cutaneous melanoma. J Clin Oncol. 2014;32:9074. doi: 10.1200/jco.2014.32.15_suppl.9074. [DOI] [Google Scholar]
- 13.Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, DeLuca HF. Vitamin D 25-hydroxylase - Four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523:30–36. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Jones G, Prosser D, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014;55:13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christiakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–29. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wacker M, Holic MF. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bikle DD. Vitamin D: an ancient hormone. Exp Dermatol. 2011;1:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 20.Hossein-Nezhad A, Holick MF. Vitamin D for health: A global perspective. Mayo Clinic Proceedings. 2013;88:720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 23.Caccamo, D., Ricca, S., Currò, M. & Ientile, R. Health risks of hypovitaminosis D: A review of new molecular insights. Int J Mol Sci, 19, 10.3390/ijms19030892 (2018). [DOI] [PMC free article] [PubMed]
- 24.Fleet JC, Desmet M, Johnson R, Li Y. Vitamin D and Cancer: A review of molecular mechanisms. Biochem J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 26.Slominski AT, et al. On the role of classical and novel forms of vitamin D in melanoma progression and management. J Steroid Biochem Mol Biol. 2018;177:159–170. doi: 10.1016/j.jsbmb.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slominski AT, et al. Vitamin D signaling and melanoma: Role of Vitamin D and its receptors in melanoma progression and management. Lab Investig. 2017;97:706–724. doi: 10.1038/labinvest.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brozyna AA, Jozwicki W, Slominski AT, Decreased VDR. expression in cutaneous melanomas as marker of tumor progression: New data and analyses. Anticancer Res. 2014;34:2735–2744. [PMC free article] [PubMed] [Google Scholar]
- 29.Jozwicki W, Brozyna A, Siekiera J, Slominski A. Expression of Vitamin D Receptor (VDR) Positively Correlates with Survival of Urothelial Bladder Cancer Patients. Int J Mol Sci. 2017;16:24369–24386. doi: 10.3390/ijms161024369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edrei R, Kimel S. Oxygen depletion during in vitro photodynamic therapy: structure-activity relationships of sulfonated aluminum phthalocyanines. J Photochem Photobiol. 1999;50:197–203. doi: 10.1016/S1011-1344(99)00092-5. [DOI] [PubMed] [Google Scholar]
- 31.Brozyna AA, Jozwicki W, Jochymski C, Slominski AT. Decreased expression of CYP27B1 correlates with the increased aggressiveness of ovarian carcinomas. Oncol Rep. 2015;33:559–606. doi: 10.3892/or.2014.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu M.-J., Zhang Q., Liang L., Wang S.-Y., Zheng X.-C., Zhou M.-M., Yang Y.-W., Zhong Q., Huang F. Association between vitamin D deficiency and risk of thyroid cancer: a case–control study and a meta-analysis. Journal of Endocrinological Investigation. 2018;41(10):1199–1210. doi: 10.1007/s40618-018-0853-9. [DOI] [PubMed] [Google Scholar]
- 33.Pereira F, Larriba MJ, Muñoz A. Vitamin D and colon cancer. Endocr Relat Cancer. 2012;19:R51–71. doi: 10.1530/ERC-11-0388. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Wang S, Che X, Li X. Vitamin D and lung cancer risk: A comprehensive review and meta-analysis. Cell Physiol Biochem. 2015;36:299–305. doi: 10.1159/000374072. [DOI] [PubMed] [Google Scholar]
- 35.Der T, et al. Vitamin D and Prostate Cancer Survival in Veterans. Mil Med. 2014;179:81–84. doi: 10.7205/MILMED-D-12-00540. [DOI] [PubMed] [Google Scholar]
- 36.Barreto SG, Neale RE. Vitamin D and pancreatic cancer. Cancer Lett. 2015;368:1–6. doi: 10.1016/j.canlet.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y, Je Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: A meta-analysis. Br J Cancer. 2014;110:2772–2784. doi: 10.1038/bjc.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jee Donghyun, Kang Seungbum, Yuan Changzheng, Cho Eunyoung, Arroyo Jorge G. Serum 25-Hydroxyvitamin D Levels and Dry Eye Syndrome: Differential Effects of Vitamin D on Ocular Diseases. PLOS ONE. 2016;11(2):e0149294. doi: 10.1371/journal.pone.0149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reins RY, McDermott AM. Vitamin D: Implications for ocular disease and therapeutic potential. Exp Eye Res. 2015;134:101–110. doi: 10.1016/j.exer.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng YF, Lu J, Xing Q, Tao JJ, Xiao P. Lower Serum Vitamin D Level Was Associated with Risk of Dry Eye Syndrome. Med Sci Monit. 2017;23:2211–2216. doi: 10.12659/msm.901857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reins Rose Y., Hanlon Samuel D., Magadi Sri, McDermott Alison M. Effects of Topically Applied Vitamin D during Corneal Wound Healing. PLOS ONE. 2016;11(4):e0152889. doi: 10.1371/journal.pone.0152889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Layana A, et al. Vitamin D and Age-Related Macular Degeneration. Nutrients. 2017;9:1120. doi: 10.3390/nu9101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alsalem JA, et al. Characterization of vitamin D production by human ocular barrier cells. Investig Ophthalmol Vis Sci. 2014;55:2140–2147. doi: 10.1167/iovs.13-13019. [DOI] [PubMed] [Google Scholar]
- 44.Solt LA, Burris TP. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol Metab. 2012;23:619–627. doi: 10.1016/j.tem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. Crystal structure of the human RORalpha Ligand binding domain in complex with cholesterol sulfate at 2.2 A. J Biol Chem. 2004;279:14033–8. doi: 10.1074/jbc.M400302200. [DOI] [PubMed] [Google Scholar]
- 46.Kallen JA, et al. X-ray structure of the hRORα LBD at 1.63 Å: Structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORα. Structure. 2002;10:1697–1707. doi: 10.1016/S0969-2126(02)00912-7. [DOI] [PubMed] [Google Scholar]
- 47.Slominski AT, et al. RORα and RORγ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–2789. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slominski AT, et al. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORα and RORγ. J Steroid Biochem Mol Biol. 2017;173:42–56. doi: 10.1016/j.jsbmb.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slominski, A. T. et al. Characterization of a new pathway that activates lumisterol invivo to biologically active hydroxylumisterols. Sci Rep7, 10.1038/s41598-017-10202-7 (2017). [DOI] [PMC free article] [PubMed]
- 50.Jetten Anton M. Retinoid-Related Orphan Receptors (RORs): Critical Roles in Development, Immunity, Circadian Rhythm, and Cellular Metabolism. Nuclear Receptor Signaling. 2009;7(1):nrs.07003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jetten AM, Jetten MER. Possible role of retinoic acid binding protein in retinoid stimulation of embryonal carcinoma cell differentiation. Nature. 1979;278:180–2. doi: 10.1038/278180a0. [DOI] [PubMed] [Google Scholar]
- 52.Jetten AM, Joo JH. Retinoid-related orphan receptors (RORs): Roles in cellular differentiation and development. Adv Dev Biol. 2006;16:313–355. doi: 10.1016/S1574-3349(06)16010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jetten AM, Euda E. Retinoid-related orphan receptors (RORs): Roles in cell survival, differentation and disease. Cell Death Differ. 2002;9:1167–1171. doi: 10.1038/sj.cdd.4401085. [DOI] [PubMed] [Google Scholar]
- 54.Jetten AM, Takeda Y, Slominski A, Kang HS. Retinoic acid-related orphan receptor γ (RORγ): Connecting sterol metabolism to regulation of the immune system and autoimmune disease. Curr Opin Toxicol. 2018;8:66–80. doi: 10.1016/j.cotox.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Z, et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 56.Kurebayashi S, et al. Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci USA. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Andrew C., Tran Hien G., Zhang Eric E., Priest Aaron A., Welsh David K., Kay Steve A. Redundant Function of REV-ERBα and β and Non-Essential Role for Bmal1 Cycling in Transcriptional Regulation of Intracellular Circadian Rhythms. PLoS Genetics. 2008;4(2):e1000023. doi: 10.1371/journal.pgen.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeda Y, Birault V, Jetten AM. The Retinoic acid-related Orphan Receptor γ (RORγ) directly regulates the circadian expression of clock genes and downstream targets in vivo. Nucleic Acids Res. 2012;40:8519–8535. doi: 10.1093/nar/gks630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slominski AT, et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slominski AT, et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 2015;5:14875. doi: 10.1038/srep14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slominski AT, et al. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 2014;383:181–192. doi: 10.1016/j.mce.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brozyna, A. A., Jozwicki, W., Skobowiat, C., Jetten, A. & Slominski, A. T. RORα and RORγ expression inversely correlates with human melanoma progression. Oncotarget10.18632/oncotarget.11211 (2016). [DOI] [PMC free article] [PubMed]
- 63.Al-Azhri J, et al. Tumor Expression of Vitamin D Receptor and Breast Cancer Histopathological Characteristics and Prognosis. Clin Cancer Res. 2015;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bises G, et al. 25-Hydroxyvitamin D3-1α-hydroxylase Expression in Normal and Malignant Human Colon. J Histochem Cytochem. 2004;52:985–989. doi: 10.1369/jhc.4B6271.2004. [DOI] [PubMed] [Google Scholar]
- 65.Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum Pathol. 2001;42:618–631. doi: 10.1016/j.humpath.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brozyna AA, et al. CYP24A1 expression inversely correlates with melanoma progression: Clinic-pathological studies. Int J Mol Sci. 2014;15:19000–19017. doi: 10.3390/ijms151019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1) decreases during melanoma progression. Hum Pathol. 2013;44:374–387. doi: 10.1016/j.humpath.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janjetovic Z, et al. High basal NF-kB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–1884. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kivelä Tero. Clinical Ophthalmic Oncology. 2007. Uveal malignant melanoma: histopathologic features; pp. 219–225. [Google Scholar]
- 70.Slominski A, et al. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch Biochem Biophys. 2014;563:79–93. doi: 10.1016/j.abb.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slominski AT, Carlson JA. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clin Proc. 2014;89:429–433. doi: 10.1016/j.mayocp.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slominski RM, Zmijewski MA, Slominski AT. The role of melanin pigment in melanoma. Exp Dermatol. 2015;24:258–259. doi: 10.1111/exd.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sniegocka M, et al. Transplantable melanomas in hamsters and gerbils as models for human melanoma. Sensitization in melanoma radiotherapy - From animal models to clinical trials. Int J Mol Sci. 2018;19:1048. doi: 10.3390/ijms19041048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slominski A, Paus R, Mihm M. Inhibition of melanogenesis as an adjuvant strategy for the treatment of melanotic melanomas. Selective review and hypothesis. Anticancer Res. 1998;18:3709–3716. [PubMed] [Google Scholar]
- 75.Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124:1470–1477. doi: 10.1002/ijc.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 77.Slominski A, Zmijewski M, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edge, S., Byrd, D. & Compton, C. Malignant melanoma of the uvea. AJCC Cancer Staging Manual. Springer: New York, USA, pp. 547–559 (2010).
- 79.Brozyna AA, Jozwicki W, Carlson JA, Slominski AT. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum Pathol. 2013;44:2071–4. doi: 10.1016/j.humpath.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]