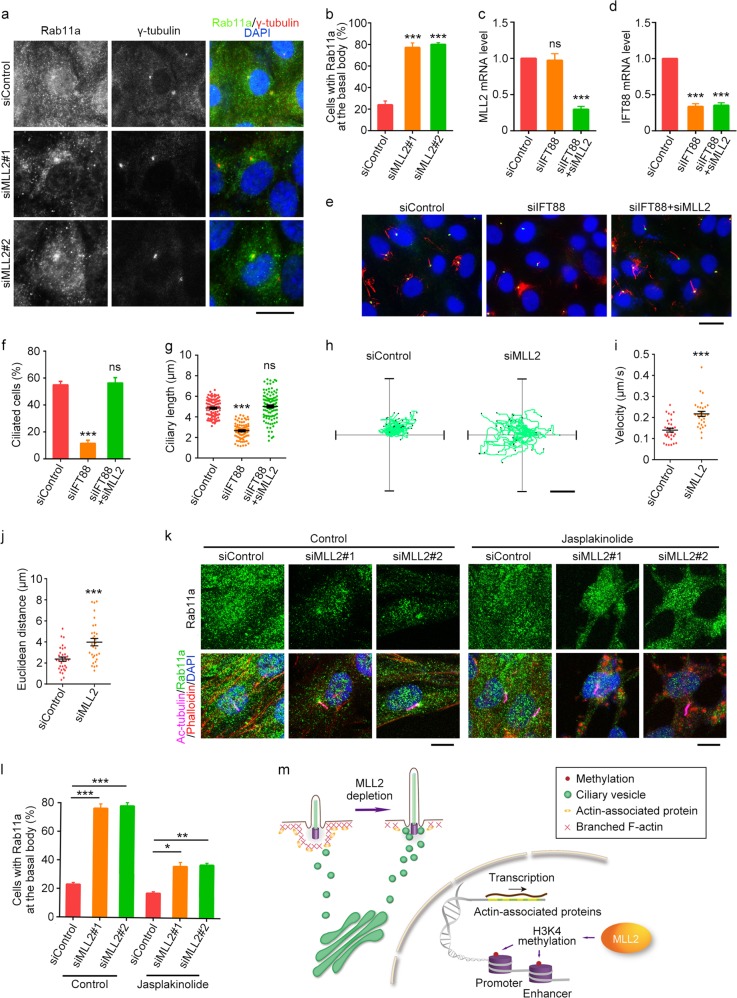
Fig. 6. Knockdown of MLL2 expression stimulates the transport of ciliary vesicles to the basal body.
a, b Immunofluorescence images (a) and percentage of cells with Rab11a at the basal body (b, n = 100) for RPE-1 cells transfected with control or MLL2 siRNAs, serum-starved for 24 h, and stained with antibodies against Rab11a (green) and γ-tubulin (red), and DAPI (blue). Scale bar, 10 µm. c, d Quantitative RT-PCR analysis of the relative mRNA levels of MLL2 (c) and IFT88 (d) for RPE-1 cells transfected with the indicated siRNAs and serum-starved for 48 h. e–g Immunofluorescence images (e), percentage of ciliated cells (f, n = 200), and ciliary length (g, n = 80) for RPE-1 cells transfected with the indicated siRNAs and serum-starved for 24 h. Cells were stained with antibodies against γ-tubulin (green) and acetylated α-tubulin (red), and DAPI (blue). Scale bar, 10 µm. h–j Movement trajectories (h), velocity (i), and Euclidean distance (j) of GFP-Rab11a vesicles in RPE-1 cells transfected with GFP-Rab11a and control or MLL2 siRNAs and serum-starved for 24 h (n = 32 for siControl; n = 29 for siMLL2). Scale bar, 4 µm. k, l Immunofluorescence images (k) and percentage of cells with Rab11a at the basal body (l, n = 100) for RPE-1 cells transfected with control or MLL2 siRNAs, serum-starved for 24 h, and treated with jasplakinolide (50 nM) for 24 h. Cells were stained with antibodies against Rab11a (green) and acetylated α-tubulin (red), and DAPI (blue). Scale bar, 5 µm. m Molecular model for the role of MLL2 in ciliary regulation. MLL2 depletion downregulates actin-associated proteins, disrupts branched F-actin, and stimulates the transport of ciliary vesicles to the basal body, thereby promoting ciliogenesis. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant. Error bars indicate SEM