Table 1.
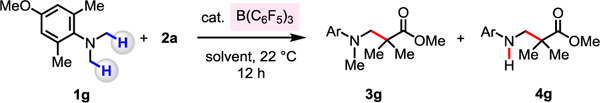 | |||||
|---|---|---|---|---|---|
| entry | Lewis acid | catalyst loading (%) | solvent | yield (%) | |
| 3g | 4g | ||||
| 1 | B(C6F5)3 | 20 | DCE | 56 | 35 |
| 2 | none | 0 | DCE | 0 | 0 |
| 3 | B(C6F5)3 | 10 | DCE | 71 | 22 |
| 4 | B(C6F5)3 | 10 | Et2O | 83 | 17 |
| 5 | B(C6F5)3 | 5.0 | Et2O | 22 | <5 |
| 6 | B(C6F5)3 | 10 | THF | 38 | <5 |
| 7 | B(C6F5)3 | 10 | toluene | 75 | 21 |
| 8 | B(C6F5)3 | 10 | benzene | 81 | 16 |
| 9 | B(C6F5)3 | 5.0 | benzene | >95 | <5 |
| 10 | BF3•OEt2 | 10 | benzene | 0 | 0 |
| 11 | BPh3 | 10 | benzene | 0 | 0 |
Conditions: 4-methoxy-N,N,2,6-tetramethylaniline (0.1 mmol), 1-methoxy-2-methyl-1-(trimethylsiloxy)propene (0.2 mmol), Lewis acid, solvent (0.25 mL), under N2, 22 °C, 12 h.
Yields were determined by 1H NMR analysis of unpurified product mixtures with mesitylene as the internal standard. See the Supporting Information for details.
