Buruli ulcer is treatable with antibiotics. An 8-week course of rifampin (RIF) and either streptomycin (STR) or clarithromycin (CLR) cures over 90% of patients.
KEYWORDS: Buruli ulcer, Mycobacterium ulcerans, Q203, bedaquiline, clofazimine, mouse footpad, rifampin, rifapentine, streptomycin, telacebec
ABSTRACT
Buruli ulcer is treatable with antibiotics. An 8-week course of rifampin (RIF) and either streptomycin (STR) or clarithromycin (CLR) cures over 90% of patients. However, STR requires injections and may be toxic, and CLR shares an adverse drug-drug interaction with RIF and may be poorly tolerated. Studies in a mouse footpad infection model showed that increasing the dose of RIF or using the long-acting rifamycin rifapentine (RPT), in combination with clofazimine (CFZ), a relatively well-tolerated antibiotic, can shorten treatment to 4 weeks. CFZ is reduced by a component of the electron transport chain (ETC) to produce reactive oxygen species toxic to bacteria. Synergistic activity of CFZ with other ETC-targeting drugs, the ATP synthase inhibitor bedaquiline (BDQ) and the bc1:aa3 oxidase inhibitor Q203 (now named telacebec), was recently described against Mycobacterium tuberculosis. Recognizing that M. tuberculosis mutants lacking the alternative bd oxidase are hypersusceptible to Q203 and that Mycobacterium ulcerans is a natural bd oxidase-deficient mutant, we tested the in vitro susceptibility of M. ulcerans to Q203 and evaluated the treatment-shortening potential of novel 3- and 4-drug regimens combining RPT, CFZ, Q203, and/or BDQ in a mouse footpad model. The MIC of Q203 was extremely low (0.000075 to 0.00015 μg/ml). Footpad swelling decreased more rapidly in mice treated with Q203-containing regimens than in mice treated with RIF and STR (RIF+STR) and RPT and CFZ (RPT+CFZ). Nearly all footpads were culture negative after only 2 weeks of treatment with regimens containing RPT, CFZ, and Q203. No relapse was detected after only 2 weeks of treatment in mice treated with any of the Q203-containing regimens. In contrast, 15% of mice receiving RIF+STR for 4 weeks relapsed. We conclude that it may be possible to cure patients with Buruli ulcer in 14 days or less using Q203-containing regimens rather than currently recommended 56-day regimens.
INTRODUCTION
Buruli ulcer is currently treated, as per WHO recommendations, with an 8-week regimen, as follows: rifampin (RIF) at 10 mg/kg of body weight together with either 15 mg/kg streptomycin (STR) or, more recently, 15 to 30 mg/kg clarithromycin (CLR) (1). RIF+CLR is an all-oral regimen that does not require a health care worker to administer it or the patient to endure 56 intramuscular injections of STR or the potential toxicity of STR (2). CLR also has drawbacks in terms of gastrointestinal tolerability (3, 4) and a drug-drug interaction with RIF that drastically reduces CLR exposures (5–7). Both regimens require 2 months of treatment, often resulting in patient or provider nonadherence. We recently reported (8) that treatment can be shortened to 4 weeks in a mouse model of Buruli ulcer by increasing the dose of RIF, or a long-acting rifamycin, rifapentine (RPT), together with clofazimine (CFZ), but even shorter treatment is desirable.
The electron transport chain (ETC) and oxidative phosphorylation have recently proven to be vulnerable targets for exciting new drugs for tuberculosis (TB) (9). The ATP synthase inhibitor bedaquiline (BDQ) has significant treatment-shortening effects in mouse TB models and is a core component of novel treatment-shortening regimens for both drug-susceptible and multidrug-resistant TB (10–16). The cytochrome bc1:aa3 complex inhibitor Q203 has efficacy in mouse TB models and is poised to enter phase 2 trials in TB patients (17, 18). Clofazimine is reduced by NDH-2 (type II dehydrogenase), which is the point of entry of electrons into the respiratory chain (9, 19). Another exciting development is the recent in vitro finding of substantial synergistic effects of CFZ with either BDQ or Q203 against Mycobacterium tuberculosis and optimal effects when all 3 inhibitors are combined (20). We hypothesized that combining Q203 and/or BDQ with CFZ, with or without high-dose rifamycin, has the potential for even greater treatment shortening in Buruli ulcer. Mycobacterium ulcerans may be particularly susceptible to combinations of drugs acting on the ETC because the alternative, but less efficient, cytochrome bd oxidase may help alleviate to some extent the stressful effects of BDQ and Q203 on the ETC in M. tuberculosis (20); also, M. ulcerans is naturally bd oxidase deficient due to a mutation in cydA (MUL_1604) resulting in a pseudogene (21, 22). Similar M. tuberculosis mutants (i.e., those lacking cydA and cydB) are hypersusceptible to Q203 (17).
Here, we investigated whether the inclusion of Q203 (now designated telacebec by its developer, Qurient Co., Ltd.) in different combination treatment regimens containing drugs targeting the ETC results in rapid declines in footpad swelling and bacterial burden, as well as the prevention of relapse after treatment discontinuation in a mouse model of Buruli ulcer. We found that regimens with Q203 reduced swelling and bacterial burden 2 weeks faster than in mice treated with either RIF+STR or with RPT+CFZ. Regimens containing RPT+CFZ+Q203 rendered mouse footpads stably culture negative after only 2 weeks of treatment.
RESULTS
MICs of study drugs.
The MICs of the study drugs (in micrograms per milliliter) against our M. ulcerans strains, Mu1615 and Mu1059, were 0.00015 and 0.000075, respectively, for Q203, 0.125 for BDQ, 1.0 for CFZ, and 0.06 to 0.125 for RPT for both strains.
Infection and treatment initiation.
Mice were infected with 4.51 log10 CFU M. ulcerans 1059 per footpad, which resulted in an implantation of 3.38 ± 0.23 log10 CFU when assessed the following day. Treatment was initiated 46 days (∼6.5 weeks) after infection, when the mean footpad swelling index was 2.91 ± 0.31 (median, 3.0) on a scale of 0 to 4 (23).
Response to treatment.
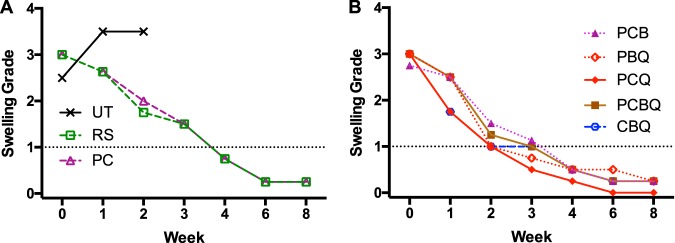
(i) Footpad swelling during treatment. Whereas footpad swelling continued to increase in untreated mice from a mean of 2.5 to 3.5 by week 1, swelling in mice treated with the RIF+STR and RPT+CFZ regimens declined from 2.83 and 2.86 to 2.58 and 2.63, respectively. There were similar declines in mice treated with RPT+CFZ+BDQ, RPT+BDQ+Q203, and RPT+CFZ+BDQ+Q203. The declines in mice treated with RPT+CFZ+Q203 or CFZ+BDQ+Q203 were somewhat greater and were statistically significantly different (P ≤ 0.0002 and <0.0005, respectively) from either the RIF+STR or RPT+CFZ regimen (see Table S3 in the supplemental material). By week 2, swelling grades declined to 1.75 and 2.3 in the RIF+STR and RPT+CFZ control regimens, respectively, whereas the mean swelling grades were 1.18 or less in all the Q203-containing regimens, except for the 4-drug RPT+CFZ+BDQ+Q203 regimen, in which mean swelling was slightly higher at 1.27 (P < 0.0001 compared to RIF+STR and RPT+CFZ). In the test regimen lacking Q203, i.e., RPT+CFZ+BDQ, there was mean swelling grade of 1.54 (P < 0.03 compared to RIF+STR; P < 0.0001 compared to RPT+CFZ). At week 3, mean swelling continued to decline in the control regimens, RIF+STR and RPT+CFZ, to 1.50, while mean swelling was 1.15 in the RPT+CFZ+BDQ group and 1.07 or less in all the Q203-containing regimen groups (P ≤ 0.0001 compared to either control regimen). By week 4, mice in all groups had mean swelling of <1.00, although there was considerable variability in the footpads of the control regimen mice. By week 6, the variability was diminished, and the mean swelling in all groups was <0.50. Compared to the 4-drug regimen, only RPT+CFZ+Q203 was significantly (P ≤ 0.0008) more active in reducing footpad swelling at all time points, indicating antagonism of the BDQ component based on this endpoint. In summary, mean swelling declined about 2 weeks more rapidly in the footpads of mice receiving a Q203-containing regimen (Fig. 1).
FIG 1.
Effect of treatment on footpad swelling. (A and B) Swelling grades in untreated (UT) mice and mice receiving a control regimen, RS (RIF+STR) or PC (RPT+CFZ) (A), or mice receiving test regimens with drugs targeting the electron transport chain, as follows C (CFZ), Q203 (Q) and/or BDQ (B) with or without P (RPT) (B). The dotted line indicates a swelling grade of 1; values below 1 may be a surrogate for successful treatment. Medians only are shown for clarity; mean and standard deviations are shown in Table S2 and in Fig. S1. Compared to RS- and PC-treated mice, swelling grades were statistically significantly lower in footpads of mice treated with PCQ or CBQ at week 1 and in all Q-containing regimens at week 2 and at week 3. See Table S3 for all statistical test results.
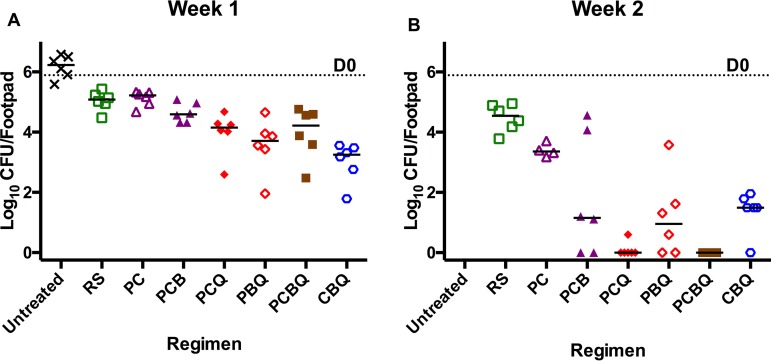
(ii) Bacterial burden during treatment. In untreated mice, the mean CFU increased from day 0 to week 1 from 5.89 ± 0.22 to 6.17 ± 0.38. Mean CFU values declined in the first week by nearly 1 log10 in the RIF+STR and RPT+CFZ groups to 5.04 ± 0.32 and 5.12 ± 0.26, respectively. In the test regimen without Q203, i.e., RPT+CFZ+BDQ, the mean CFU count was 4.65 ± 0.32. In all the Q203-containing regimens, the reduction was much greater, with CFU counts below 4 log10 in all four of the groups RPT+CFZ+Q203, RPT+BDQ+Q203, RPT+CFZ+BDQ+Q203, and CFZ+BDQ+Q203, with differences in means approaching or achieving statistical significance compared to RIF+STR (P ≤ 0.05; Table S3g) and achieving statistical significance compared to RPT+CFZ (P < 0.03; Table S3o). At week 2, the CFU counts showed further reductions in the control RIF+STR (4.48 ± 0.46) and RPT+CFZ (3.40 ± 0.22) groups comparable to that observed in previous experiments. Mice treated with RPT+CFZ+BDQ showed a steeper decline to 1.82 ± 2.00 (P = 0.0006 compared to RIF+STR but P > 0.1 compared to RPT+CFZ), while the Q203 groups again declined still further to 0.10 ± 0.25 for RPT+CFZ+Q203, 1.19 ± 1.35 for RPT+BDQ+Q203, 0.00 ± 0.00 for RPT+CFZ+BDQ+Q203, and 1.37 ± 0.70 for CFZ+BDQ+Q203. The mean CFU counts were significantly lower than with RIF+STR for all Q203-containing regimens (P < 0.0001; Table S3h) and compared to those of RPT+CFZ (P < 0.04; Table S3p). Deletion of Q203 from the 4-drug regimen resulted in significantly higher (P < 0.04; Table S3x) CFU counts in the footpads of mice treated with the RPT+CFZ+BDQ regimen. By week 4, with the exception of mice treated with RIF+STR (0.10 ± 0.25), all mice were culture negative (Fig. 2).
FIG 2.
Effect of treatment on M. ulcerans burden in mouse footpads. CFU counts after 1 week (A) and 2 weeks (B) of treatment with the different control, RS (RIF+STR) or PC (RPT+CFZ), and test regimens: PCB (RPT+CFZ+BDQ), PCQ (RPT+CFZ+Q203), PBQ (RPT+BDQ+Q203), PCBQ (RPT+CFZ+BDQ+Q203), and CBQ (CFZ+BDQ+Q203). The dotted line indicates the mean log10 CFU count at the beginning of treatment (D0). Bars represent the median CFU counts. The Q-containing regimens were significantly more active in reducing the bacterial burden compared to RS and PC at week 1 and at week 2. See Table S3 for all statistical test results.
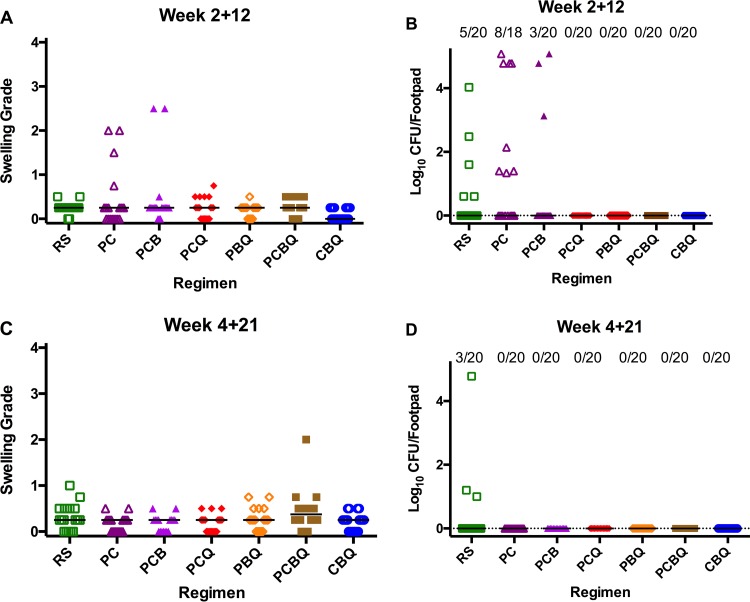
(iii) Footpad swelling and bacterial burden at the relapse assessment. At the first relapse time point where mice had been treated for 2 weeks and then left without treatment for the next 12 weeks (2 + 12), swelling remained in 2 of 20 footpads in mice treated with RPT+CFZ+BDQ. Both swollen footpads were observed in the same mouse, and both had a swelling grade of 2.5. Swelling was also observed in four of 18 footpads from two of nine mice treated with RPT+CFZ; the swelling grades for these footpads were 1.5 and 2.0 in one mouse and 2.0 and 0.75 in the other. In all other groups, residual swelling, defined as grade 0.5 or less, or no swelling was observed, with the exception of one footpad in a mouse treated with RPT+CFZ+Q203 that was grade 0.75 (Fig. 3A).
FIG 3.
Relapse after treatment completion. (A and B) Swelling grades (A) and M. ulcerans CFU counts (B) in mouse footpads after 2 weeks of treatment and 12 weeks without treatment. (C and D) Swelling (C) and M. ulcerans CFU counts (D) after 4 weeks of treatment and 21 weeks without treatment. Bars represent median swelling grade or CFU count. (B and D) The proportion relapsing with cultivable M. ulcerans is indicated at top of each graph. R, RIF; S, STR; P, RPT; C, CFZ; B, BDQ; Q, Q203. The differences in proportions relapsing at both time points did not reach statistical significance (P = 0.0967 between the PC group and most other groups). At week 2 + 12, the bacterial burden was statistically significantly greater in the PC group than in any Q-containing group (P = 0.0002) and compared to RS (P = 0.0241) but was not different from that in the PCB group. At week 4 + 21, the bacterial burden was significantly higher in the RS group than in all other groups (P = 0.0426).
In terms of bacterial burden at week 2 + 12, CFU were found in 5 of 20 footpads from 3 mice treated with RIF+STR. There were 4.03 log10 CFU in the mouse with a single positive footpad; in a second mouse, there were 2.48 and 1.60 log10 CFU found in the two footpads, while a single CFU was found in each footpad from a third mouse. In the RPT+CFZ-treated mice, CFU were detected in 8 of 18 footpads, and the counts ranged from 1.34 to approximately 5.08 log10 per footpad. In the RPT+CFZ+BDQ-treated mice, CFU were detected in 3 of 20 footpads, and CFU counts ranged from 3.13 to approximately 5.08 log10. The correlation between footpad swelling and CFU counts was high in the RPT+CFZ and the RPT+CFZ+BDQ groups and statistically significant at P values of 0.0005 and 0.0002, respectively, but was not significant (P = 0.19) in the RIF+STR group. No CFU were detected in any of the mice treated with a Q203-containing regimen (Fig. 3B). Thus, the proportion of mice relapsing after receiving 2 weeks of any Q203-containing regimen was significantly lower than that in the RIF+STR (P < 0.05) and RPT+CFZ (P < 0.001) groups treated for the same duration.
To confirm the superior curative effect of Q203-containing regimens after even longer durations of follow-up without treatment, we extended the follow-up period from 12 to 21 to 22 weeks in mice treated for 4 to 6 weeks. At week 4 + 21, only residual swelling was observed in the following groups: RPT+CFZ, RPT+CFZ+BDQ, RPT+CFZ+Q203, and CFZ+BDQ+Q203. In RIF+STR-treated mice, one mouse had grade 1.0 swelling in one footpad, and another mouse had grade 0.75 swelling in one footpad. In the RPT+BDQ+Q203 group, a single mouse had grade 0.75 swelling in both footpads. In the RPT+CFZ+BDQ+Q203 group, one mouse had grade 2.0 and 0.75 swelling in its footpads, and another mouse had grade 0.75 swelling in one footpad. The median swelling grade was 0.25 in all groups except for the RPT+CFZ+BDQ+Q203 group, in which it was 0.375 due to the one footpad with high swelling (Fig. 3C).
At the week 4 + 21 relapse time point, CFU were detected only in 3 of 20 footpads from 3 mice in the RIF+STR group and in none of the other groups. In two RIF+STR-treated footpads, there were only a few colonies detected. However, in the third footpad, the colonies were too numerous to count precisely (Fig. 3D). PCR and sequencing analyses of these colonies detected no mutations in the rpoB rifampin-resistance determining region. There were no detectable CFU in mice treated with RIF+STR for 6 weeks and left untreated for 22 weeks (data not shown).
DISCUSSION
The ETC and oxidative phosphorylation are targets of two new antituberculosis drugs, BDQ and Q203, while CFZ is a recently rediscovered antituberculosis drug that also disrupts the ETC by siphoning electrons and transferring them to O2 to generate reactive oxygen species (ROS) via a redox cycling mechanism (19). A potential mechanism for their synergistic activity against M. tuberculosis was recently proposed. BDQ targets ATP synthase (24), resulting in the inhibition of ATP production. Q203 inhibits the bc1:aa3 oxidase complex leading to the rerouting of electrons to the alternative, less-efficient bd oxidase in M. tuberculosis. Increased ETC flux resulting from a compensatory response to Q203 and/or BDQ exposure results in an increased rate of one-electron reduction of O2 and enhanced production of toxic ROS (20). Because M. tuberculosis cydA mutants are hypersusceptible to Q203 and “classical” M. ulcerans strains present in the regions of greatest endemicity of West Africa and Australia also have a mutated cydA resulting in a nonfunctional bd oxidase (22, 25), we hypothesized that M. ulcerans would be even more susceptible to the effects of Q203 alone or combined with BDQ and/or CFZ than M. tuberculosis.
Rifamycin drugs such as RIF and RPT have been found to be well tolerated at higher, more effective doses in clinical trials for TB (26–28) (ClinicalTrials.gov identifiers NCT02581527, NCT03474198, and NCT02410772). We have previously shown that these higher doses, together with CFZ (8) or clarithromycin (29), are able to reduce treatment time by half in the mouse footpad model of Buruli ulcer. Therefore, we also incorporated high-dose RPT into the 3- and 4-drug combinations tested in the current study.
Shortly before the completion of the current experiments, a report appeared showing the potency of Q203 against M. ulcerans in vitro and in mice (22). Consistent with our hypothesis, and like the classical strains recently studied by Scherr et al. (22), our M. ulcerans strains that were originally clinical isolates from Ghana and Malaysia were exquisitely sensitive to Q203 in vitro. The results of the present experiment also show, for the first time, that Q203 has treatment-shortening potential when used in combination therapy for the treatment of Buruli ulcer. RPT+CFZ is a sterilizing regimen that rendered footpads culture negative after 4, but not 2, weeks of treatment in the mouse footpad model. Strikingly, adding Q203 to RPT+CFZ led to a further shortening of the treatment duration needed to achieve culture negativity to 2 weeks in 5 of 6 mouse footpads. Culture negativity was also achieved after 2 weeks in all mice treated with RPT+CFZ+BDQ+Q203 but not in mice treated with only CFZ, BDQ, and Q203, suggesting that the inclusion of (high-dose) rifamycin is also critical to optimizing bactericidal activity. Substitution of BDQ for either Q203 or CFZ failed to render most mice culture negative after 2 weeks of treatment. The inclusion of Q203 in regimens with any two other companion drugs for 2 weeks was sufficient to prevent relapse in all footpads (Fig. 2B). The failure to render footpads culture negative at week 2 while preventing relapse 12 weeks later despite no further dosing indicates that there may be persistent drug effects related to tissue accumulation or postantibiotic effects associated with Q203 either alone or in combination with the other drugs. Alternatively, host immunity after reduction of mycobacterial burden and the accompanying inhibition of mycolactone production (30, 31), as seen in the mouse model as well as in patients who have relatively smaller, less severe lesions (32–34), may also account for the absence of relapse. Small lesions in patients, unlike the advanced ones in the mouse model, sometimes heal spontaneously (35).
In this study, we built on our recent experience with RPT+CFZ as a backbone for a novel treatment-shortening regimen (8). However, both RPT and CFZ have limited clinical availability at this time. Ongoing experiments are assessing whether higher doses of RIF, a drug already in use clinically in Buruli ulcer treatment, in combination with Q203 would comprise a simpler, more readily available regimen with comparable efficacy.
Examination of mycobacterial genomes (genodb.pasteur.fr, accessed from July 2018 to April 2019, currently available at PRJNA16230) indicated that QcrB, the target of Q203 and component of the terminal bc1:aa3 oxidase, is highly conserved across the genus Mycobacterium. CydA is also very well conserved in the genus, with the exception of M. ulcerans and M. leprae. In M. ulcerans, cydA is deemed to be a pseudogene (21), while in M. leprae, cydB, cydD, and cydC are all absent (36). Scherr et al. (22) showed that cydA is intact in the M. ulcerans subsp. shinshuense (a subspecies found in all or nearly all Japanese patients) genome (37) and that it is also functional. The gene is also intact in Mycobacterium marinum, thought to be the species from which all M. ulcerans evolved. The MIC is higher in the “ancestral” M. ulcerans subsp. shinshuense strain than in the classical strains found in Africa and Australia (22, 25). Scherr et al. (22) also reported that, as in other mycobacterial cydA genes, base 692 is G in M. ulcerans subsp. shinshuense as part of a TGG codon encoding tryptophan, whereas in classical M. ulcerans strains, there is a truncating TAG stop codon at amino acid 231 in the 486-amino-acid mycobacterial protein. Their results imply that Q203 may not be a useful option in Japanese patients.
In summary, we have shown that combinations of drugs attacking the electron transport chain and that have all advanced beyond phase 1 safety testing in humans are highly active against M. ulcerans in the mouse footpad model. Regimens containing Q203 may reduce treatment time from 8 weeks, as required currently, to 2 weeks or less and may warrant clinical study.
MATERIALS AND METHODS
Bacteria.
M. ulcerans 1059 (Mu1059), originally obtained from a patient in Ghana, was generously provided by Pamela Small, University of Tennessee. This strain produces mycolactone A/B, and this toxin kills macrophages and fibroblasts in vitro (38, 39). The Mu1059 strain was passaged in mouse footpads before use in these studies. The bacilli were harvested from footpads with grade 2 level swelling, i.e., swelling with inflammation (23). M. ulcerans 1615 (Mu1615) was also provided by Pamela Small and was originally obtained from a Malaysian patient.
Ethics statement.
All animal procedures were conducted according to relevant national and international guidelines. The study was conducted in adherence to the Johns Hopkins University guidelines for animal husbandry and was approved by the Johns Hopkins Animal Care and Use Committee. Johns Hopkins University is in compliance with the Animal Welfare Act regulations and Public Health Service Policy and also maintains accreditation of its program by the private Association for the Assessment and Accreditation of Laboratory Animal Care International.
Antibiotics.
CFZ, RIF, and STR were purchased from Sigma (St. Louis, MO). RPT was prepared from Priftin (Sanofi) tablets purchased at a pharmacy. BDQ and Q203 were provided by the Global Alliance of TB Drug Development (New York, NY). Stock RIF, RPT, and CFZ suspensions were prepared weekly in a sterile 0.05% (wt/vol) agarose solution in distilled water; the STR solution was prepared weekly in water. BDQ was prepared in acidified 20% hydroxypropyl-β-cyclodextrin. Q203 was formulated in 20% (wt/wt) d-α tocopheryl polyethylene glycol 1000 succinate solution and was administered by gavage. All drugs were given 5 days per week in 0.2 ml. Doses of 25 mg/kg BDQ, 12.5 mg/kg CFZ, 10 mg/kg Q203, 10 mg/kg RIF, and 20 mg/kg RPT were administered by oral gavage. STR (150 mg/kg) was administered by subcutaneous injection (Table S1). The doses were the same as those used in previous studies in our lab and sought to match mean plasma exposures (i.e., area under the concentration-time curve over 24 h postdose) with human doses (8, 30, 40).
Determination of MICs of study drugs.
Previously frozen broth cultures (optical density at 600 nm [OD600], 0.1) of two strains of M. ulcerans (Mu1615 and Mu1059) diluted 1:10 in 1× sterile phosphate-buffered saline (PBS) were used as the inocula. Complete 7H11 agar medium was prepared with serial 2-fold dilutions of the respective antibiotics. Plates without the drug were used as a control. Half a milliliter of serial 10-fold dilutions of the above-described inocula were plated onto drug-free and drug-containing plates. The plates were incubated for 10 weeks at 32°C before final CFU counts were determined. The lowest drug concentration that inhibited 99% of the growth of the inoculum was considered the MIC.
Infection and efficacy analysis.
BALB/c mice (n = 228), age ∼6 weeks (Charles River Laboratories, Wilmington, MA), were inoculated in both hind footpads with approximately 4.51 log10 CFU of mouse-passaged Mu1059 in 0.03 ml PBS, resulting in a mean (± standard deviation [SD]) CFU count of 3.38 ± 0.23 log10 M. ulcerans per footpad the day after infection. Treatment began 46 days after infection when footpad swelling increased to approximately grade 3 on a scale from 0 to 4 (23). Treatment with RPT+CFZ, RPT+CFZ+BDQ, RPT+CFZ+Q203, RPT+BDQ+Q203, RPT+CFZ+BDQ+Q203, or CFZ+BDQ+Q203 was administered for up to 4 weeks or up to 6 weeks with RIF+STR. All rifamycins were administered at least 4 h before other drugs in the combinations. Footpads were harvested before treatment initiation (day 0) and after 1, 2, 4, and 6 weeks of treatment from mice (6 footpads from 3 mice) for CFU counts. For relapse determinations, 10 mice (20 footpads) were held without treatment for at least 12 weeks after completing a 2-, 4-, or 6-week combination regimen treatment (see the overall experiment scheme, including each regimen evaluated, in Table S1). Mice were euthanized if a footpad exceeded grade 3 swelling (23). Footpad tissue was harvested, minced with fine scissors, suspended in 1.5 ml PBS, serially diluted, and plated on Middlebrook selective 7H11 plates (Becton, Dickinson, Sparks, MD). Plates were incubated at 32°C, and colonies were counted after 8 to 12 weeks of incubation.
Rifamycin resistance analysis.
Genomic DNA from individual colonies isolated at the week 4 + 21 relapse time point was extracted by boiling in 1× Tris-EDTA (TE [pH 8.0]) buffer for 5 min; 5 μl of the supernatant was then used for PCR. Specific primers, forward primer MU_rpoF 5′-CGACGACATCGACCACTTC-3′ and reverse primer MU_rpoR 5′-CGACAGTGAACCGATCAGAC-3′, were used to amplify a 400-bp region encompassing the rifampin resistance-determining region. The PCR product was then sequenced to identify the presence of any mutation. Colonies from untreated control groups were used as a negative control.
Statistical analysis.
GraphPad Prism 6 was used to compare group means by one-way analysis of variance with Bonferroni’s posttest to assess the correlation of footpad swelling and CFU counts with the Spearman r test, as well as to compare group proportions by Fisher’s exact test.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by grant R01-AI-113266 from the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00426-19.
REFERENCES
- 1.World Health Organization. 2017. Report from the Meeting of the Buruli ulcer Technical Advisory Group. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Klis S, Stienstra Y, Phillips RO, Abass KM, Tuah W, van der Werf TS. 2014. Long term streptomycin toxicity in the treatment of buruli ulcer: follow-up of participants in the BURULICO drug trial. PLoS Negl Trop Dis 8:e2739. doi: 10.1371/journal.pntd.0002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien DP, Friedman D, Hughes A, Walton A, Athan E. 2017. Antibiotic complications during the treatment of Mycobacterium ulcerans disease in Australian patients. Intern Med J 47:1011–1019. doi: 10.1111/imj.13511. [DOI] [PubMed] [Google Scholar]
- 4.Williams KN, Bishai WR. 2005. Clarithromycin extended-release in community-acquired respiratory tract infections. Expert Opin Pharmacother 6:2867–2876. doi: 10.1517/14656566.6.16.2867. [DOI] [PubMed] [Google Scholar]
- 5.Koh WJ, Jeong BH, Jeon K, Lee SY, Shin SJ. 2012. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 186:797–802. doi: 10.1164/rccm.201206-1088OC. [DOI] [PubMed] [Google Scholar]
- 6.van Ingen J, Egelund EF, Levin A, Totten SE, Boeree MJ, Mouton JW, Aarnoutse RE, Heifets LB, Peloquin CA, Daley CL. 2012. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med 186:559–565. doi: 10.1164/rccm.201204-0682OC. [DOI] [PubMed] [Google Scholar]
- 7.Wallace RJ Jr, Brown BA, Griffith DE, Girard W, Tanaka K. 1995. Reduced serum levels of clarithromycin in patients treated with multidrug regimens including rifampin or rifabutin for Mycobacterium avium-M. intracellulare infection. J Infect Dis 171:747–750. doi: 10.1093/infdis/171.3.747. [DOI] [PubMed] [Google Scholar]
- 8.Converse PJ, Almeida DV, Tasneen R, Saini V, Tyagi S, Ammerman NC, Li S-Y, Anders NM, Rudek MA, Grosset JH, Nuermberger EL. 2018. Shorter-course treatment for Mycobacterium ulcerans disease with high-dose rifamycins and clofazimine in a mouse model of Buruli ulcer. PLoS Negl Trop Dis 12:e0006728. doi: 10.1371/journal.pntd.0006728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bald D, Villellas C, Lu P, Koul A. 2017. Targeting energy metabolism in Mycobacterium tuberculosis, a new paradigm in antimycobacterial drug discovery. mBio 8:e00272-17. doi: 10.1128/mBio.00272-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Hutchings J, Burger DA, Schall R, Mendel CM. 2015. Bactericidal activity of pyrazinamide and clofazimine alone and in combinations with pretomanid and bedaquiline. Am J Respir Crit Care Med 191:943–953. doi: 10.1164/rccm.201410-1801OC. [DOI] [PubMed] [Google Scholar]
- 11.Diacon AH, Pym A, Grobusch MP, de los Rios JM, Gotuzzo E, Vasilyeva I, Leimane V, Andries K, Bakare N, De Marez T, Haxaire-Theeuwes M, Lounis N, Meyvisch P, De Paepe E, van Heeswijk RPG, Dannemann B. 2014. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 371:723–732. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 12.Horsburgh CR, Barry CE, Lange C. 2015. Treatment of tuberculosis. N Engl J Med 373:2149–2160. doi: 10.1056/NEJMra1413919. [DOI] [PubMed] [Google Scholar]
- 13.Lanoix J-P, Betoudji F, Nuermberger E. 2014. Novel regimens identified in mice for treatment of latent tuberculosis infection in contacts of patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 58:2316–2321. doi: 10.1128/AAC.02658-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tasneen R, Betoudji F, Tyagi S, Li S-Y, Williams K, Converse PJ, Dartois V, Yang T, Mendel CM, Mdluli KE, Nuermberger EL. 2016. Contribution of oxazolidinones to the efficacy of novel regimens containing bedaquiline and pretomanid in a mouse model of tuberculosis. Antimicrob Agents Chemother 60:270–277. doi: 10.1128/AAC.01691-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasneen R, Li S-Y, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56:3114–3120. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalia NP, Hasenoehrl EJ, Ab Rahman NB, Koh VH, Ang MLT, Sajorda DR, Hards K, Gruber G, Alonso S, Cook GM, Berney M, Pethe K. 2017. Exploiting the synthetic lethality between terminal respiratory oxidases to kill Mycobacterium tuberculosis and clear host infection. Proc Natl Acad Sci U S A 114:7426–7431. doi: 10.1073/pnas.1706139114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, Christophe T, Lee H, Kempf M, Jackson M, Lenaerts AJ, Pham H, Jones V, Seo MJ, Kim YM, Seo M, Seo JJ, Park D, Ko Y, Choi I, Kim R, Kim SY, Lim S, Yim S-A, Nam J, Kang H, Kwon H, Oh C-T, Cho Y, Jang Y, Kim J, Chua A, Tan BH, Nanjundappa MB, Rao SPS, Barnes WS, Wintjens R, Walker JR, Alonso S, Lee S, Kim J, Oh S, Oh T, Nehrbass U, Han S-J, No Z, et al. 2013. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med 19:1157. doi: 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 19.Yano T, Kassovska-Bratinova S, Teh JS, Winkler J, Sullivan K, Isaacs A, Schechter NM, Rubin H. 2011. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase. J Biol Chem 286:10276–10287. doi: 10.1074/jbc.M110.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamprecht DA, Finin PM, Rahman MA, Cumming BM, Russell SL, Jonnala SR, Adamson JH, Steyn AJ. 2016. Turning the respiratory flexibility of Mycobacterium tuberculosis against itself. Nat Commun 7:12393. doi: 10.1038/ncomms12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, Meurice G, Simon D, Bouchier C, Ma L, Tichit M, Porter JL, Ryan J, Johnson PD, Davies JK, Jenkin GA, Small PL, Jones LM, Tekaia F, Laval F, Daffe M, Parkhill J, Cole ST. 2007. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res 17:192–200. doi: 10.1101/gr.5942807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherr N, Bieri R, Thomas SS, Chauffour A, Kalia NP, Schneide P, Ruf MT, Lamelas A, Manimekalai MSS, Gruber G, Ishii N, Suzuki K, Tanner M, Moraski GC, Miller MJ, Witschel M, Jarlier V, Pluschke G, Pethe K. 2018. Targeting the Mycobacterium ulcerans cytochrome bc1:aa3 for the treatment of Buruli ulcer. Nat Commun 9:5370. doi: 10.1038/s41467-018-07804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dega H, Bentoucha A, Robert J, Jarlier V, Grosset J. 2002. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob Agents Chemother 46:3193–3196. doi: 10.1128/AAC.46.10.3193-3196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 25.Doig KD, Holt KE, Fyfe JA, Lavender CJ, Eddyani M, Portaels F, Yeboah-Manu D, Pluschke G, Seemann T, Stinear TP. 2012. On the origin of Mycobacterium ulcerans, the causative agent of Buruli ulcer. BMC Genomics 13:258. doi: 10.1186/1471-2164-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, Kibiki GS, Churchyard G, Sanne I, Ntinginya NE, Minja LT, Hunt RD, Charalambous S, Hanekom M, Semvua HH, Mpagama SG, Manyama C, Mtafya B, Reither K, Wallis RS, Venter A, Narunsky K, Mekota A, Henne S, Colbers A, van Balen GP, Gillespie SH, Phillips PPJ, Hoelscher M. 2017. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 17:39–49. doi: 10.1016/S1473-3099(16)30274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dian S, Yunivita V, Ganiem AR, Pramaesya T, Chaidir L, Wahyudi K, Achmad TH, Colbers A, Te Brake L, van Crevel R, Ruslami R, Aarnoutse R. 2018. Double-blind, randomized, placebo-controlled phase II dose-finding study to evaluate high-dose rifampin for tuberculous meningitis. Antimicrob Agents Chemother 62:e01014-18. doi: 10.1128/AAC.01014-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorman SE, Savic RM, Goldberg S, Stout JE, Schluger N, Muzanyi G, Johnson JL, Nahid P, Hecker EJ, Heilig CM, Bozeman L, Feng PJ, Moro RN, MacKenzie W, Dooley KE, Nuermberger EL, Vernon A, Weiner M. 2015. Daily rifapentine for treatment of pulmonary tuberculosis. A randomized, dose-ranging trial. Am J Respir Crit Care Med 191:333–343. doi: 10.1164/rccm.201410-1843OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omansen TF, Almeida D, Converse PJ, Li SY, Lee J, Stienstra Y, van der Werf T, Grosset JH, Nuermberger EL. 2018. High-dose rifamycins enable shorter oral treatment in a murine model of Mycobacterium ulcerans disease. Antimicrob Agents Chemother 63:e01478-18. doi: 10.1128/AAC.01478-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Converse PJ, Tyagi S, Xing Y, Li SY, Kishi Y, Adamson J, Nuermberger EL, Grosset JH. 2015. Efficacy of rifampin plus clofazimine in a murine model of Mycobacterium ulcerans disease. PLoS Negl Trop Dis 9:e0003823. doi: 10.1371/journal.pntd.0003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Converse PJ, Xing Y, Kim KH, Tyagi S, Li S-Y, Almeida DV, Nuermberger EL, Grosset JH, Kishi Y. 2014. Accelerated detection of mycolactone production and response to antibiotic treatment in a mouse model of Mycobacterium ulcerans disease. PLoS Negl Trop Dis 8:e2618. doi: 10.1371/journal.pntd.0002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klis S, Kingma RA, Tuah W, van der Werf TS, Stienstra Y. 2016. Clinical outcomes of Ghanaian Buruli ulcer patients who defaulted from antimicrobial therapy. Trop Med Int Health 21:1191–1196. doi: 10.1111/tmi.12745. [DOI] [PubMed] [Google Scholar]
- 33.Nienhuis WA, Stienstra Y, Thompson WA, Awuah PC, Abass KM, Tuah W, Awua-Boateng NY, Ampadu EO, Siegmund V, Schouten JP, Adjei O, Bretzel G, van der Werf TS. 2010. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375:664–672. doi: 10.1016/S0140-6736(09)61962-0. [DOI] [PubMed] [Google Scholar]
- 34.Phillips RO, Sarfo FS, Abass MK, Abotsi J, Wilson T, Forson M, Amoako YA, Thompson W, Asiedu K, Wansbrough-Jones M. 2014. Clinical and bacteriological efficacy of rifampin-streptomycin combination for two weeks followed by rifampin and clarithromycin for six weeks for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother 58:1161–1166. doi: 10.1128/AAC.02165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Brien DP, Murrie A, Meggyesy P, Priestley J, Rajcoomar A, Athan E. 2019. Spontaneous healing of Mycobacterium ulcerans disease in Australian patients. PLoS Negl Trop Dis 13:e0007178. doi: 10.1371/journal.pntd.0007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honore N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida M, Nakanaga K, Ogura Y, Toyoda A, Ooka T, Kazumi Y, Mitarai S, Ishii N, Hayashi T, Hoshino Y. 2016. Complete genome sequence of Mycobacterium ulcerans subsp. shinshuense. Genome Announc 4:e01050-16. doi: 10.1128/genomeA.01050-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Converse PJ, Almeida DV, Nuermberger EL, Grosset JH. 2011. BCG-mediated protection against Mycobacterium ulcerans infection in the mouse. PLoS Negl Trop Dis 5:e985. doi: 10.1371/journal.pntd.0000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang T, Li S-Y, Converse PJ, Almeida DV, Grosset JH, Nuermberger EL. 2011. Using bioluminescence to monitor treatment response in real time in mice with Mycobacterium ulcerans infection. Antimicrob Agents Chemother 55:56–61. doi: 10.1128/AAC.01260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida D, Converse PJ, Ahmad Z, Dooley KE, Nuermberger EL, Grosset JH. 2011. Activities of rifampin, rifapentine and clarithromycin alone and in combination against Mycobacterium ulcerans Disease in Mice. PLoS Negl Trop Dis 5:e933. doi: 10.1371/journal.pntd.0000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.