The activities of rifampin, nitazoxanide, PA-824, and sutezolid were tested against dormant Mycobacterium tuberculosis under conditions mimicking caseous granulomas (hypoxia at pH 7.3) in comparison with those of the combination rifampin-isoniazid-pyrazinamide-ethambutol (R-I-Z-E), which is used for human therapy. Mycobacterial viability was monitored by CFU and regrowth in MGIT 960.
KEYWORDS: Mycobacterium tuberculosis, hypoxia, nitazoxanide, pH, rifampin, tuberculosis
ABSTRACT
The activities of rifampin, nitazoxanide, PA-824, and sutezolid were tested against dormant Mycobacterium tuberculosis under conditions mimicking caseous granulomas (hypoxia at pH 7.3) in comparison with those of the combination rifampin-isoniazid-pyrazinamide-ethambutol (R-I-Z-E), which is used for human therapy. Mycobacterial viability was monitored by CFU and regrowth in MGIT 960. As shown by lack of regrowth in MGIT, rifampin-nitazoxanide-containing combinations, but not R-I-Z-E, killed dormant cells in 28 to 35 days. These observations might be important in designing new tuberculosis therapies.
TEXT
Mycobacterium tuberculosis is responsible for about 10 million new tuberculosis (TB) cases per year. Furthermore, approximately 1.7 billion people are latently infected with this organism and 10% of them reactivate to active TB in their lifetime (1). Current antibiotic treatments require 6 months of combination therapy with rifampin (R), isoniazid (I), pyrazinamide (Z), and ethambutol (E) (R-I-Z-E) for active TB and at least 6 months of I or 3 to 4 months of R-I for latent TB (2). Shortening the duration of therapy could increase adherence to treatment and reduce development of drug-resistant TB.
The lungs of patients with active and latent TB contain cellular and caseous granulomas, with M. tuberculosis bacilli ranging from actively replicating (AR) to dormant, nonreplicating (NR) stages (3). In caseous granulomas, the reduced vascularization and low oxygen pressure restricts the growth of AR to NR stages in their necrotic, hypoxic (H) centers, allowing M. tuberculosis to transit into a dormant, extracellular, drug-refractory state. The pH of necrotic TB cavities is 7.2 to 7.5 in C3HeB/FeJ mice (4), 7.0 to 7.5 in guinea pigs, and 6.4 to 7.4 in rabbits (5). In a recent paper on human TB lung tissues, two lesions showed pH 7.2 (severe necrosis) and eight showed a pH of ≤5.5 (rare to severe necrosis) (6).
The caseum has no vascular supply and in M. tuberculosis-infected rabbits, the fraction unbound (fu) of a drug penetrates the caseum via passive diffusion (7). Caseum binding of a drug is proportional to hydrophobicity (cLogP) and aromatic ring count. The chance of penetrating caseum is high for compounds with low cLogP values; for instance, drugs with a cLogP of <2 had a 34 to 67% chance to reach an fu of >10% (7).
M. tuberculosis is extremely tolerant to drugs in caseum, with rifamycins being the only agents sterilizing caseum obtained from rabbits (8). Using a Wayne dormancy culture model of hypoxia at pH 7.3, we found a similar trend of phenotypic resistance, with R and rifapentine highly effective in killing M. tuberculosis and with no or little activity by other drugs (9, 10). These observations suggest that this model could mimic caseum to measure drug activity against NR M. tuberculosis bacilli in this environment.
Rifampin accumulates slowly in caseum and maintains therapeutic levels throughout the dosing intervals (7). Here, the hypoxia model at pH 7.3 was used to measure the activity of R-containing combinations against NR bacilli in caseum-mimicking conditions compared with that of R-I-Z-E, currently used for TB therapy.
Briefly, for preparation of NR, hypoxic (H) cells, M. tuberculosis H37Rv log-phase cultures were diluted in Dubos-Tween-albumin broth (DTAB) adjusted to pH 7.3 (10, 11) and incubated at 37°C for 12 days (H12) in 20- by 125-mm stirred tubes (120 rpm) containing 16 ml of culture (0.5 headspace ratio) (9) with the caps tightly screwed and tight rubber caps put under the screw caps. Control tubes with 1.5 μg/ml of methylene blue as an indicator of oxygen depletion were also incubated. Noticeable decolorization of methylene blue was observed around day 12. Drugs were added by syringe to H12 cultures at their maximum concentration of drug in serum (Cmax) (μg/ml) (10, 12, 13) as follows: R, 8; I, 2; E, 4; PA-824 (PA), 2; nitazoxanide (NZ), 10; sutezolid (SZ), 1. Pyrazinamide was used at 100 μg/ml. Every week, 1 ml of NR culture was washed twice and resuspended in 1 ml of DTAB, and 0.2 ml was inoculated in Middlebrook 7H10 agar plates for CFU determination and in liquid medium (Bactec MGIT 960 system) for determination of the number of days to reach a growth unit of ≥75 (day to positivity [DTP]) (12). M. tuberculosis killing was defined as a lack of regrowth in MGIT after >100 days.
The drugs tested in combination with R (PA, NZ, and SZ) were chosen for having some activity against H12 cells at pH 7.3 in our previous studies (10; our unpublished data) and for their low cLogP values (PA, 2.8; NZ, 2.14; SZ, 1.31) (http://www.drugbank.ca/) predictive of caseum penetration (7).
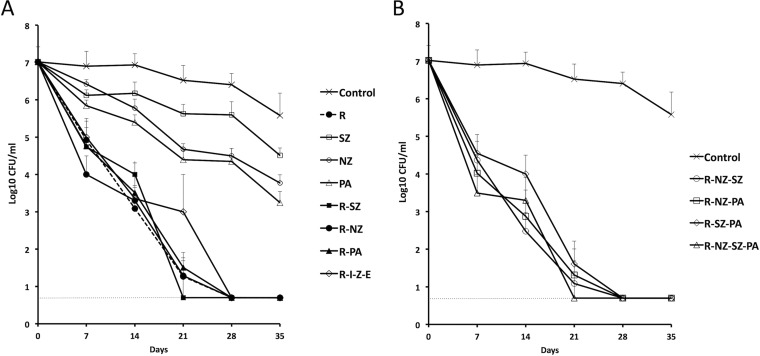
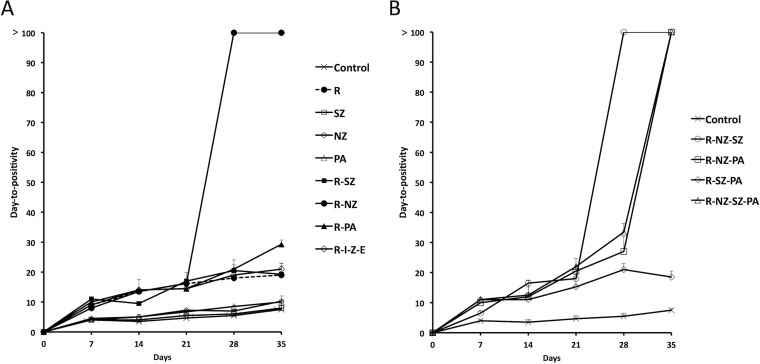
Figure 1A and B and Fig. 2A and B show the CFU and DTP values, respectively, of R, PA, NZ, SZ, alone and combined, and of the combination R-I-Z-E. On day 35, untreated control cultures showed a 1.4-log10 CFU reduction from day 0 (Fig. 1A). Among single drugs, R was much more active than PA, NZ, and SZ with a >4.9, 2.3, 1.8, and 1.1-log10 CFU reduction, respectively, compared to the day 35 control. On day 28, no CFU was detected after treatment with 2-, 3-, and 4-drug combinations (Fig. 1A and B). Since NR M. tuberculosis bacilli may not form colonies on agar (12), the samples for which CFU were determined were also inoculated in MGIT in order to measure the DTPs of surviving cells (Fig. 2A and B). On day 21, untreated or single-drug treated bacilli showed DTP values of 6 to 7 days, whereas DTPs of bacilli treated with R alone or R-containing combinations became positive at 15 to 22 days. In contrast, cells exposed to any R-NZ-containing combination were killed (DTP, >100 days) on day 28 (R-NZ, R-NZ-SZ) or 35 (R-NZ-PA, R-NZ-SZ-PA). No killing occurred on day 35 after treatment with NZ-free combinations, including R-SZ, R-PA, R-SZ-PA, and R-I-Z-E.
FIG 1.
Survival of nonreplicating (NR) cultures of M. tuberculosis after exposure to drugs (alone and in combination) as estimated by CFU counts. Twelve-day-old (H12) cultures adjusted to pH 7.3 were incubated with drugs. Rifampin (R), 8 μg/ml; sutezolid (SZ), 1 μg/ml; nitazoxanide (NZ), 10 μg/ml; PA-824 (pretomanid; PA), 2 μg/ml; isoniazid (I), 2 μg/ml; pyrazinamide (Z), 100 μg/ml; ethambutol (E), 4 μg/ml. Dashed lines indicate the limit of detection (5 CFU/ml). Means and standard deviations from two experiments are shown. In each experiment, two Wayne tubes per condition per time point were used, from which at least two 7H10 agar plates were inoculated. (A) Single drugs, 2-drug combinations, and R-I-Z-E; (B) 3- and 4-drug combinations.
FIG 2.
Days to positivity (DTP) of nonreplicating (NR) cultures of M. tuberculosis after exposure to drugs (alone and in combination) are shown. The samples (0.2 ml of diluted cultures) for which results are shown in Fig. 1A and B were also inoculated in MGIT 960 in order to measure the DTP of surviving cells. Means and standard deviations from two experiments are shown. In each experiment, two Wayne tubes per condition per time point were used, from which at least two MGIT were inoculated. (A) Single drugs, 2-drug combinations, R-I-Z-E; (B) 3- and 4-drug combinations.
These observations suggest that only NZ, but not SZ, PA, or I-Z-E, synergized with R to kill NR M. tuberculosis bacilli under caseum-mimicking conditions. The tuberculocidal activity of the couple R-NZ was also observed against H12 cells at pH 5.8 since the combinations R-NZ-moxifloxacin-amikacin and R-moxifloxacin-amikacin killed them in 14 and 21 days, respectively (12).
Nitazoxanide is a broad-spectrum antimicrobial with a good safety profile approved by the FDA for treatment of Giardia and Cryptosporidium infections (14). The drug is also active against viruses and anaerobic/microaerophilic bacteria and helminthes (14). With regards to M. tuberculosis, NZ kills AR and NR bacilli through the disruption of membrane potential and pH homeostasis (15, 16). Nitazoxanide might have multiple targets in M. tuberculosis since no resistant cells were found among >1012 CFU. Nitazoxanide is one of the seven repurposed drugs for TB undergoing further testing, and it is presently evaluated in phase II studies of early bactericidal activity (1). In anaerobic bacteria and parasites, NZ inhibits the pyruvate:ferredoxin/flavodoxin oxidoreductase (14, 17). M. tuberculosis encodes an anaerobic-type alpha-ketoglutarate ferredoxin oxidoreductase involving the Rv2454c and Rv2455c genes (18). These genes may play a role on the activity of NZ since their mRNAs were mildly overexpressed in Wayne cultures on day 16 and 40 (19).
Overall, the inability of R-I-Z-E to kill NR M. tuberculosis under caseum-mimicking conditions may provide a new framework for designing therapies that are shorter than 6 months containing R and NZ, a well-tolerated drug targeting anaerobic metabolism and likely penetrating caseum where dormant bacilli live.
A limitation of our study is that we did not supplement MGIT cultures with M. tuberculosis culture filtrates (CF), which may generate differentially culturable tubercle bacilli (DCTB) during antibiotic treatment and in the Cornell mouse model (20–22). However, the effect of the addition of CF to culture medium was variable in studies of DCTB in sputum samples, with values being enhanced in sputum samples obtained after drug treatment and decreased in sputum samples obtained before treatment or when host immunity was reduced (20, 21).
REFERENCES
- 1.World Health Organization. 2018. Global tuberculosis report WHO/CDS/TB/2018.20. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. 2017. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med 167:248–255. doi: 10.7326/M17-0609. [DOI] [PubMed] [Google Scholar]
- 3.Lenaerts A, Barry CE III, Dartois V. 2015. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev 264:288–307. doi: 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanoix JP, Lenaerts AJ, Nuermberger EL. 2015. Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Dis Model Mech 8:603–610. doi: 10.1242/dmm.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanoix JP, Ioerger T, Ormond A, Kaya F, Sacchettini J, Dartois V, Nuermberger E. 2016. Selective inactivity of pyrazinamide against tuberculosis in C3HeB/FeJ mice is best explained by neutral pH of caseum. Antimicrob Agents Chemother 60:735–743. doi: 10.1128/AAC.01370-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempker RR, Heinrichs MT, Nikolaishvili K, Sabulua I, Bablishvili N, Gogishvili S, Avaliani Z, Tukvadze N, Little B, Bernheim A, Read TD, Guarner J, Derendorf H, Peloquin CA, Blumberg HM, Vashakidze S. 2017. Lung tissue concentrations of pyrazinamide among patients with drug-resistant pulmonary tuberculosis. Antimicrob Agents Chemother 61:e00226-17. doi: 10.1128/AAC.00226-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarathy JP, Zuccotto F, Hsinpin H, Sandberg L, Via LE, Marriner GA, Masquelin T, Wyatt P, Ray P, Dartois V. 2016. Prediction of drug penetration in tuberculosis lesions. ACS Infect Dis 2:552–563. doi: 10.1021/acsinfecdis.6b00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarathy JP, Via LE, Weiner D, Blanc L, Boshoff H, Eugenin EA, Barry CE III, Dartois VA. 2018. Extreme drug tolerance of Mycobacterium tuberculosis in caseum. Antimicrob Agents Chemother 62:e02266-17. doi: 10.1128/AAC.02266-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wayne LG, Hayes LG. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun 64:2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iacobino A, Piccaro G, Giannoni F, Mustazzolu A, Fattorini L. 2017. Mycobacterium tuberculosis is selectively killed by rifampin and rifapentine in hypoxia at neutral pH. Antimicrob Agents Chemother 61:e02296-16. doi: 10.1128/AAC.02296-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacobino A, Piccaro G, Giannoni F, Mustazzolu A, Fattorini L. 2017. Fighting tuberculosis by drugs targeting nonreplicating Mycobacterium tuberculosis bacilli. Int J Mycobacteriol 6:213–221. doi: 10.4103/ijmy.ijmy_85_17. [DOI] [PubMed] [Google Scholar]
- 12.Piccaro G, Giannoni F, Filippini P, Mustazzolu A, Fattorini L. 2013. Activities of drug combinations against Mycobacterium tuberculosis grown in aerobic and hypoxic acidic conditions. Antimicrob Agents Chemother 57:1428–1433. doi: 10.1128/AAC.02154-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallis RS, Dawson R, Friedrich SO, Venter A, Paige D, Zhu T, Silvia A, Gobey J, Ellery C, Zhang Y, Eisenach K, Miller P, Diacon AH. 2014. Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis. PLoS One 9:e94462. doi: 10.1371/journal.pone.0094462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakya A, Bhat HR, Ghosh SK. 2018. Update on nitazoxanide: a multifunctional chemotherapeutic agent. Curr Drug Discov Technol 15:201–213. doi: 10.2174/1570163814666170727130003. [DOI] [PubMed] [Google Scholar]
- 15.de Carvalho LP, Lin G, Jiang X, Nathan C. 2009. Nitazoxanide kills replicating and nonreplicating Mycobacterium tuberculosis and evades resistance. J Med Chem 52:5789–5792. doi: 10.1021/jm9010719. [DOI] [PubMed] [Google Scholar]
- 16.de Carvalho LP, Darby CM, Rhee KY, Nathan C. 2011. Nitazoxanide disrupts membrane potential and intrabacterial pH homeostasis of Mycobacterium tuberculosis. ACS Med Chem Lett 2:849–854. doi: 10.1021/ml200157f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. 2007. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob Agents Chemother 51:868–876. doi: 10.1128/AAC.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baughn AD, Garforth SJ, Vilchèze C, Jacobs WR Jr.. 2009. An anaerobic-type alpha-ketoglutarate ferredoxin oxidoreductase completes the oxidative tricarboxylic acid cycle of Mycobacterium tuberculosis. PLoS Pathog 5:e1000662. doi: 10.1371/journal.ppat.1000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iona E, Pardini M, Mustazzolu A, Piccaro G, Nisini R, Fattorini L, Giannoni F. 2016. Mycobacterium tuberculosis gene expression at different stages of hypoxia-induced dormancy and upon resuscitation. J Microbiol 54:565–572. doi: 10.1007/s12275-016-6150-4. [DOI] [PubMed] [Google Scholar]
- 20.McAulay K, Saito K, Warrier T, Walsh KF, Mathurin LD, Royal-Mardi G, Lee MH, Ocheretina O, Pape JW, Fitzgerald DW, Nathan CF. 2018. Differentially detectable Mycobacterium tuberculosis cells in sputum from treatment-naive subjects in Haiti and their proportionate increase after initiation of treatment. mBio 9:e02192-18. doi: 10.1128/mBio.02192-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chengalroyen MD, Beukes GM, Gordhan BG, Streicher EM, Churchyard G, Hafner R, Warren R, Otwombe K, Martinson N, Kana BD. 2016. Detection and quantification of differentially culturable tubercle bacteria in sputum from patients with tuberculosis. Am J Respir Crit Care Med 194:1532–1540. doi: 10.1164/rccm.201604-0769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y, Liu A, Ortega-Muro F, Alameda-Martin L, Mitchison D, Coates A. 2015. High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front Microbiol 6:641. doi: 10.3389/fmicb.2015.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]