Abstract
Background.
Uterine sarcomas are rare gynaecologic tumours representing 3–7% of all uterine malignancies. The aetiology of sarcomas is still unclear: it is thought, that chromosomal translocations have influence on wide histological variety of sarcomas. Presenting symptoms are vague and nonspecific. Usually sarcoma causes abnormal vaginal bleeding, can cause abdominal or pelvic pain, or manifests as a rapidly growing uterine tumour. The diagnosis of sarcoma is often made retrospectively after surgical removal of a presumed benign uterine neoplasm, because imaging modalities such as ultrasound, computed tomography, or magnetic resonance imaging cannot yet accurately and reliably distinguish between benign leiomyoma and malignant pathology. If there are certain clinical features that raise a suspicion of malignancy in the uterus, it is recommended to avoid the use of power morcellation through laparoscopic surgery in order to prevent disease dissemination.
Materials and methods
We present a clinical case of a 64-year-old patient, who was referred to hospital due to abdominal pain and tenesmus that lasted for two days. From a past medical history it was known that previously the patient had been diagnosed with uterine myoma. Transvaginal ultrasonography showed a 10.4 cm × 9.8 cm uterine tumour of nonhomogeneous structure with signs of necrosis and good vascularization. The patient refused urgent hysterectomy, that was advised to her. The patient was operated on one month later and total hysterectomy with bilateral salpingooforectomy was performed. Postoperative histological evaluation showed undifferentiated sarcoma uterus pT1b L/V0. Imaging modalities were made to evaluate possible dissemination of the disease. In the absence of signs of disease progression, the patient received radiotherapy and brachytherapy and was followed-up by doctors.
Results and conclusions
Uterine sarcomas are highly malignant tumours that originate from smooth muscles and connective tissue elements of the uterus and make up 1% of all malignant gynaecological tumours and about 3–7% of all malignant uterine tumours. Imaging modalities cannot yet reliably distinguish benign myomas from malignant sarcomas. It is important not to damage the wholeness of uterus during operation in order to prevent dissemination of the disease in the abdominal cavity. The low-grade endometrial stromal sarcoma has the best survival prognosis, while carcinosarcoma and undifferentiated uterine sarcoma have the lowest survival rates.
Keywords: uterine tumour, malignant uterine neoplasm, uterine sarcoma, undifferentiated uterine sarcoma, uterine sarcoma diagnosis, treatment
Abstract
GIMDOS SARKOMA: KLINIKINIS ATVEJIS IR LITERATŪROS APŽVALGA
Santrauka
Tikslas. Gimdos sarkomos yra reti ginekologiniai navikai, sudarantys 3–7 % visų piktybinių gimdos navikų. Sarkomų atsiradimo etiologija nėra aiški, manoma, kad įtakos tam turi skirtingos genų ir chromosomų mutacijos, lemiančios didelę sarkomų histologinę įvairovę. Simptomatika nėra specifiška tik sarkomoms, šie navikai dažniausiai sukelia nenormalius kraujavimus iš gimdos, neaiškius pilvo ir dubens skausmus ar gali pasireikšti greitu gimdos darinio augimu. Diagnozė dažniausiai nustatoma po operacijos, pašalinus iki tol gerybiniu laikytą gimdos darinį ir ištyrus histologiškai, nes ikioperaciniai vaizdiniai tyrimai negali patikimai atskirti gerybinių gimdos lejomiomų nuo piktybinių sarkomų. Jeigu tiriant pastebimi požymiai, kad gali būti gimdos sarkoma, reikėtų vengti tokias pacientes operuoti naudojant morceliatorių operaciniams dariniams smulkinti, nes kyla grėsmė, kad piktybiniai dariniai išsisės pilvo ertmėje. Straipsnio tikslas – konkretaus klinikinio atvejo pavyzdžiu aprašyti diagnostikos ir gydymo metodus nustačius gimdos naviką moteriai po menopauzės.
Medžiaga ir metodai. Pristatome 64 metų pacientės klinikinį atvejį, kuri atvyko į ligoninę dėl dvi paras besitęsiančių pilvo skausmų ir pasunkėjusio tuštinimosi. Iš anamnezės buvo žinoma, kad pacientei prieš penkerius metus ambulatoriškai diagnozuota gimdos mioma. Ultragarsiniu tyrimu per makštį pacientei nustatyta 10,4 × 9,8 cm dydžio nehomogeniškos struktūros su nekrozės požymiais, gerai vaskuliarizuota gimdos intramuralinė mioma. Ligonei dėl pilvo skausmų buvo rekomenduotas operacinis gydymas skubos tvarka, įtariant miomos mazgo nekrozę, tačiau moteris atsisakė skubios operacijos. Rekomendavus operacinį gydymą planine tvarka ir paskyrus ambulatorinį ištyrimą bei gydymą, pacientė išvyko į namus. Po vieno mėnesio ligonei buvo atlikta planinė operacija – totalinė histerektomija su abipusiais gimdos priedais. Ištyrus gimdą histologiškai buvo diagnozuota nediferencijuota gimdos sarkoma pT1b L/V0. Kadangi vaizdinių tyrimai nerodė ligos išplitimo, pacientei buvo paskirta adjuvantinė spindulinė terapija ir endovagininė brachiterapija bei rekomenduotas tolimesnis stebėjimas.
Rezultatai ir išvados. Gimdos sarkoma yra didelio piktybiškumo navikas, augantis iš gimdos lygiųjų raumenų bei jungiamojo audinio, sudarantis 1 % visų piktybinių ginekologinių navikų ir apie 3–7 % visų piktybinių gimdos navikų. Vaizdiniais tyrimais negalima patikimai atskirti gerybinių gimdos miomų nuo piktybinių gimdos sarkomų. Labai svarbu operuojant ligonę, kuriai diagnozuotas gimdos navikas, nepažeisti gimdos sienelės vientisumo norint išvengti patologinio proceso išplitimo į pilvo ertmę. Mažo laipsnio endometriumo stromos sarkomos atveju ligonių išgyvenamumo prognozė yra geriausia, o karcinosarkomos ir nediferencijuotos gimdos sarkomos atvejais – blogiausia.
Raktažodžiai: gimdos tumoras, piktybinė gimdos neoplazija, gimdos sarkoma, nediferencijuota gimdos sarkoma, gimdos sarkomos diagnostika ir gydymas
INTRODUCTION
Uterine sarcomas are rare high malignancy tumours that arise from smooth muscles and connective tissue elements and represent about 1% of all malignant gynaecologic tumours and about 3–7% of all uterine malignancies (1, 2). Three most common types of sarcomas are leiomyosarcoma, carcinosarcoma, and endometrial stromal sarcoma. Uterine sarcoma usually form de novo from the soft muscle tissue as only in very rare cases a solid tumour can develop from a uterine myoma (3). The aetiology mechanisms of uterine sarcoma still remain unclear, however, it is thought that chromosomal translocations have influence on a wide histological variety of sarcomas, so, as a result, each tumour is differently malignant and has a different response to chemotherapy. According to literature, the risk of sarcoma is higher for 50-year-old or older women; also, uterine sarcoma is twice more frequent among women of the black race (2). The diagnostics of uterine sarcoma remains difficult because presenting symptoms are nonspecific and might very often be mismatched with benign uterine myomas. There are no imaging modalities that could reliably diagnose uterine sarcoma without performing operation. The risk that a woman of the reproductive age will be diagnosed with a uterine myoma once in her lifetime is between 20–40% (4), so the clinicians are facing the challenge of how to correctly distinguish that one in many women with a uterine myoma does not have rare and malignant uterine sarcoma and how to make diagnosis without unnecessary hysterectomy or laparotomy in case of a uterine myoma. Despite radical treatment of this disease, local recurrences and distant metastases are frequent; furthermore, compared with endometrium carcinoma, the prognosis of uterine sarcoma remains poor – five year survival for stage I disease is 50–70% and for higher stages 0–20% (5).
THE CLINICAL CASE
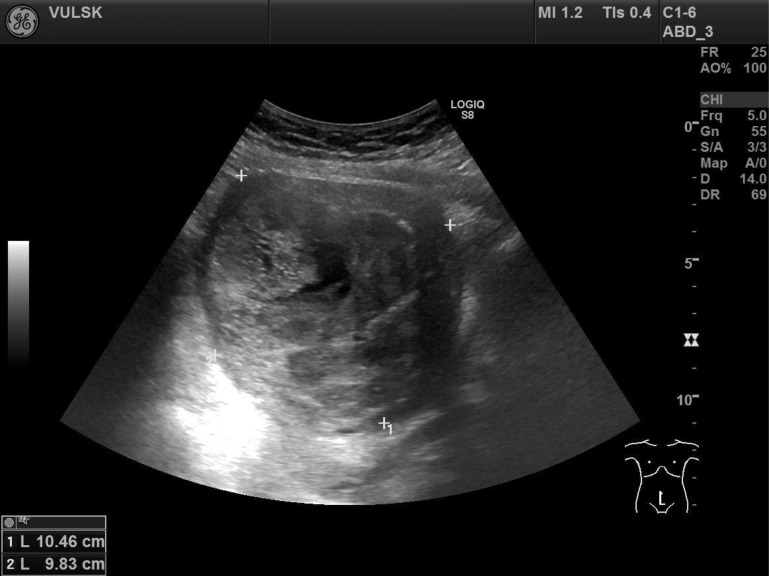
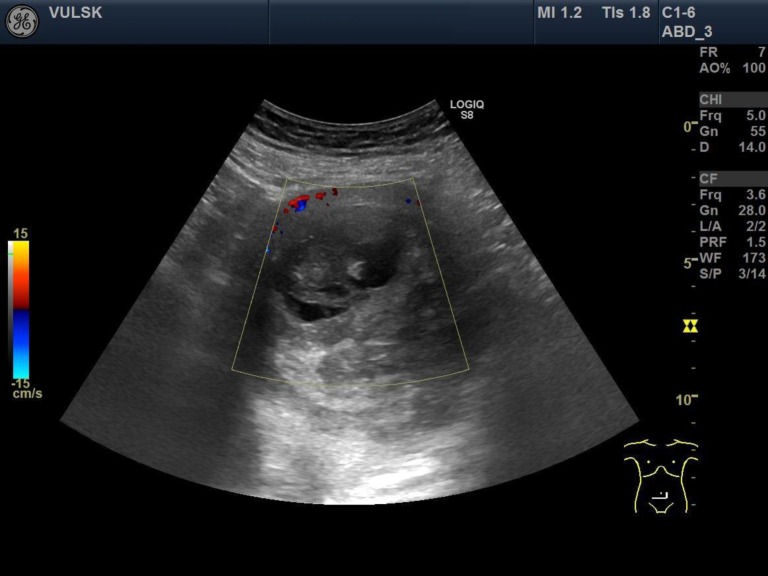
We present a case of a 64-year-old woman, who came to our hospital with complaints of abdominal pain and tenesmus that lasted for two days. From previous medical history it was known that five years ago the patient was diagnosed with a uterine myoma. She had no gynaecological operations or diseases and had already been post-menopausal for ten years. The patient’s height was 154 cm, weight 98 kg, the body mass index 41.3. Physical examination revealed a palpable normal mobility uterus with the size of 16-week pregnancy. Transvaginal ultrasonography showed a 10.4 cm × 9.8 cm uterus nodule of nonhomogeneous structure with signs of necrosis but well vascularised (Figs. 1, 2). Both ovaries were of normal size and atrophic. Laboratory tests showed an elevated level of C-reactive protein (CRB) – 165.9 mg/l. All other laboratory tests were within normal limits. In order to exclude acute abdominal diseases, the patient was consulted by an abdominal surgeon. Transabdominal ultrasound and abdomen rentgenography showed no signs of bowel perforation or ileus. After analyzing the patient’s complaints, medical history, objective and visual examination data, the preliminary diagnosis was made: abdominal pain, intramural uterine myoma. An emergent operation was recommended for the patient with the presumed diagnosis of uterine nodule necrosis, but the patient refused surgery due to personal reasons. Painkillers and antibiotics were prescribed to the patient. The operation was planned after one month. Laparotomy and total hysterectomy with bilateral salpingooopherectomy were performed. Adnexa, the urinary bladder, the appendix and bowels, and the omentum were not damaged. The uterus was about 16 weeks of pregnancy with a 10 cm nodule.
Fig. 1.
Transvaginal ultrasound – nodule of the uterus
Fig. 2.
Transvaginal ultrasound – vascularization of the uterine nodule
Postoperative histopathological evaluation showed a uterus of 14 × 10 × 11 cm in size. Macroscopically, several small white nodules of 1.7 cm in size and one lobulated nodule of 9.0 × 7.9 × 9.0 cm in size with light yellow and grey myxoid zones were seen in uterine myometrium. Microscopically, the biggest nodule consisted of spindle cells with ovoid, irregular, and polymorphic nucleus and necrosis zones up to 30% and calcificates. The mitotic count was 12/10 in the high-power field. The tumour was without intravascular dissemination. Final diagnosis was undifferentiated sarcoma of the uterus pT1b L/V0. After final diagnosis the patient was referred to the Oncology Department for chemotherapy (the patient was consulted by an oncologist-chemo-therapist). Pelvic magnetic resonance imaging, chest and abdomen computed tomography examinations were performed and showed no distant metastases. A multidisciplinary team of an obstetrician gynaecologist, an oncologist gynaecologist, and an oncologist chemotherapist) recommended adjuvant radiotherapy for the patient. The patient received external beam radiotherapy (EBRT) to the pelvis, to the upper 2/3 of vagina and regional lymph nodes. She received a median EBRT dose of 2 Gy in a median of 23 fractions, up to 46 Gy and 3 fractions of endovaginal brachytherapy up to 15 Gy. After radiotherapy, the patient’s condition was followed up every three months.
LITERATURE REVIEW AND DISCUSSION
Aetiology
Understanding the development of uterine sarcoma has been slow. It was thought that new cases of uterine sarcoma are sporadic, without any specific aetiology. However, specific chromosomal translocations have been identified in an increasing number of uterine sarcomas, resulting in fusion genes that are constitutive and involve activation of transcription factors (6). Endometrial stromal sarcoma has specific somatic mutations that have been discovered by citogenic fluorescence in situ hybridization (FISH) and polymerase chain reaction (PCR) analyses. Leiomyosarcoma also has karyotypic aberrations, but without any tumour-specific feature (6, 7). It is thought that genetic factors also play an important role in the development of uterine sarcoma, because the incidence rate is twice higher among black women than in white women (8). Some authors think that previous pelvic radiotherapy has influence on the development of leiomyosarcoma, carcinosarcoma, and undifferentiated uterus sarcoma (6, 7). Mark et al. reviewed the literature and estimated, that there existed a risk of getting leiomyosarcoma (LMS) or endometrial stromal sarcoma (ESS) after pelvic radiotherapy (median dose of 55 Gy), the risk fluctuated from 0.003% to 0.8% within a period of three to 30 years after radiotherapy (9). Hormonal therapy and tamoxifen treatment also increase the risk of uterine sarcoma. Jaakkola et al. estimated that uterine sarcoma was diagnosed for 76 women out of 243,857 who used estradiol-progestin therapy for more than six months, but despite this increased risk the absolute risk is still very low (10). Recently, it was observed that women with breast cancer, who use tamoxifen for two years or more, have a higher risk of endometrial sarcoma than the women, who do not use tamoxifen (11).
Epidemiology
The incidence rate of uterine sarcoma is 17.1 cases per one million women (12). The most common sarcoma is leiomyosarcoma, which makes up 55–70% of all cases of uterine sarcoma, carcinosarcoma – about 30%, endometrial stromal sarcoma – 20%, undifferentiated sarcoma uterus – 10%, and adenosarcoma – about 5% (2, 13, 14). Abeler et al. analysed histopathological results of Norwegian women from the period of 1970 to 2000. During this period, 419 uterine sarcomas were diagnosed in total, leiomyosarcomas came up to 62%, endometrial stromal sarcoma 20%, undifferentiated uterine sarcoma 6%, adenosarcomas 5.5%, while carcinosarcoma was not included in the study (15).
Classification
According to the 2014 classification of the World Health Association, uterine sarcomas are classified into two categories depending on the tissue of the origin: mesenchymal/mixed epithelial tumours and mesenchymal tumors. Mesenchymal tumours are leiomyosarcoma and endometrial stromal sarcoma, which is further subdivided into low-grade ESS, high-grade ESS, and undifferentiated uterine sarcoma; smooth muscle tumour of uncertain malignant potential also belong to the group of mesenchymal tumour. Carcinosarcoma and adenosarcoma belong to the group of mesenchymal/mixed epithelial tumours (16). It is important to distinguish these uterine sarcomas by their histological types in order to select appropriate treatment.
Leiomyosarcoma
It is the most common type of uterine sarcoma, which comprises about 1–2% of all malignant uterine tumours and 40–70% of all uterine sarcomas (5, 13). The prevalence is about 0.3–0.4% /100000 women per year with a higher risk for women receiving tamoxifen therapy due to breast cancer. Leiomyosarcoma is usually diagnosed for women above 50 years of age. The surface of sarcoma is typically pink or grey with necrotic or haemorrhagic sites and with irregular margins. Usually leiomyosarcomas are ≥10 cm. In about 66% of cases, sarcoma is found to be intramural, 20% submucosal, 10% subserosal, and only 5% develop in the cervix of the uterus (16). The main histological features of leiomyosarcomas are cytological atypia, mitotic activity, and necrotic zones. These leiomyosarcomas are most often composed of spindle/pleomorphic cells.
Smooth muscle tumour of uncertain malignant potential
Smooth muscle tumour of uncertain malignant potential (STUMP) is a tumour that can have malignancy features like necrosis, mitotic activity, and nuclear atypia, but it precludes an unequivocal diagnosis of leiomyosarcoma. Moreover, this tumour does not fulfill the criteria for leiomyoma, so it remains unclear if this tumour is malignant or benign. These tumours usually have good prognosis, but it is recommended to follow up patients with these disease (16).
Endometrial stromal sarcoma
Endometrial stromal sarcomas (ESS) are mesenchymal neoplasms composed of cells that morphologically resemble proliferative-phase endometrial stroma. They are classified into low-grade ESS, high-grade ESS, and into undifferentiated uterine sarcoma.
1. The second most common mesenchymal uterine sarcoma is low-grade ESS. It is usually diagnosed for 40–55-year-old women, mostly premenopausal (17). Macroscopically, soft yellow nodules can be seen that infiltrate the endometrium and the myometrium. Necrotic and haemorrhagic sites can rarely be observed. Microscopically, the tumour consists of well differentiated endometrial stromal cells with small nuclear atypia and resembles proliferative-phase endometrial stromal cells. In some literature the relation is mentioned between low-grade ESS development and polycystic ovary syndrome, oestrogen stimulation, tamoxifen therapy, and pelvic radiation in the past (16, 18, 19).
2. High-grade ESS is a rare, malignant endometrial stromal tumour. Usually it is more frequent among women between 28 and 67 years of age (16). Macroscopically, the tumour size can reach up to 9 cm and the growth is often extrauterine. Necrotic and haemorrhagic sites can be seen on the surface. Microscopically, the tumour often consists of high-grade malignant round cells with low-grade spindle cell components.
3. Undifferentiated uterine sarcoma is a very rare tumour that develops in the endometrium or the myometrium and has no morphological resemblance to proliferative-phase endometrial stroma and no specific differential type (16).
Adenosarcoma
It is a tumour the epithelial component of which is benign or atypical and the mesenchymal component is malignant. When at least 25% of the tumour contains a high-grade sarcomatous component, it is classified as an adenosarcoma with sarcomatous overgrowth. Generally, adenosarcoma can also be called Müller adenosarcoma. These tumours are typically polypoid and can fill the entire cavity of the uterus (16). If there is sarcomatous overgrowth, there is more likely to be a myometrial invasion and the tumours tend to be larger with a fleshy, haemorrhagic and necrotic cut surface (16, 18). It is observed that the incidence of adenosarcoma is higher after pelvic radiotherapy, long-term oestrogen therapy, and especially after tamoxifen therapy. Women with this disease there may have a history of uterine polyps which, on review, may be reinterpreted as adenosarcoma.
Carcinosarcoma
This tumour consists of high-grade carcinomatous and sarcomatous elements. Pathologists now believe that carcinosarcomas (also known as a mixed malignant Müller tumour) are metaplastic carcinomas and not uterine sarcomas; however, due to aggressive growth, which is normally not common for endometrial carcinoma, carcinosarcomas are grouped as separate mixed epithelial and mesenchymal tumour in 2014 World Health Association classification (16). Usually carcinosarcoma develops in women in their 70s. These tumours make up less than 5% of all uterine malignancies. As in the case of adenosarcoma, such factors in anamnesis as tamoxifen therapy, longtime oestrogen use, pelvic radiation 10–20 years before increase the risk for carcinosarcomas. Macroscopically, the tumour looks like a large, soft, and polypoid mass, filling the entire uterine cavity and often protruding through the cervical os (19). Carcinosarcoma very often grows into the myometrium and sometimes even to the cervix of the uterus.
Symptoms
Uterine sarcomas cause symptoms that are nonspecific for this disease and often may be wrongly reinterpreted as uterine myoma. Usually, sarcomas cause abdominal or pelvic pain, abdominal distension and most commonly vaginal bleeding. Also, due to malignancy the enlarged uterus can compress other internal organs like the urinary bladder or bowels, then urinary retention, bowel obstruction, obstipation, and tenesmus may begin. There are certain clinical features that should raise suspicion, such as a rapidly growing fibroid during a 3-month period in a peri- or postmenopausal woman who do not use hormone replacement therapy. Often during clinical examination the clinician may observe an enlarged uterus. Rarely, uterine sarcomas do not cause any symptoms and first signs of disease are respiratory system disorders due to metastasis in the lungs. Also, about half of patients with carcinosarcomas and a few with adenosarcomas experience tumour prolapse through cervix to vagina (19).
To conclude, uterine sarcomas are usually diagnosed for women in menopause from 50 to 70 years of life (13). Fifty per cent of cases of endometrial stromal sarcoma and 30% of adenosarcomas are diagnosed in premenopausal women or even in teenagers. The patient’s complaints, anamnesis, and clinical examination are not sufficient to distinguish if the symptoms are caused by myoma or sarcoma.
Diagnostics
Preoperative diagnosis of uterine sarcoma still remains a very challenging task for clinicians, because primary symptoms are often vague and nonspecific. Therefore it is important to thoroughly investigate the patient in order to get necessary information to make a correct decision. It is advisable to start from vaginal examination. The clinician can evaluate the size, contour, and mobility of the uterus with adnexes. A fixed mass is more suggestive of a malignant neoplasm than a mobile mass. However, this is not pathognomonic, since a malignant neoplasm that has not invaded the uterine serosa may be mobile and a mass associated with endometriosis or pelvic infection may be fixed. Unfortunately, there are no examination findings that can distinguish a leiomyoma from a uterine sarcoma. However, these findings are helpful to follow changes in the uterus over time and/or to aid surgical planning. Today there are no imaging modalities that can accurately and reliably distinguish between benign leiomyoma and malignant pathology. Both leiomyoma and sarcoma look quite the same in the uterus: both form focal masses within the uterus and in both cases central necrosis may be seen. Nowadays it is thought that pelvic ultrasound examination followed by magnetic resonance imaging is the best imaging strategy for uterine sarcoma. Sonographic evaluation of a uterine mass may identify features suggestive of sarcoma: mixed echogenic and poor echogenic parts, central necrosis and colour Doppler findings of irregular vessel distribution, although many of these findings may also be occur in benign leiomyomas (20). MRI may be helpful in women with suspected sarcoma, however, it does not provide a definitive diagnosis. A scattered haemorrhagic or necrotic mass should raise a suspicion of leiomyosarcoma. These necrotic areas are seen as areas of slightly higher intensity on T1-weighted images and as heterogeneous areas on T2-weighted images. Findings in uterine leiomyosarcomas on MRI vary and include a lobulated mass of high-signal intensity on T2-weighted images, a sharply marginated mass of low-signal intensity that closely resembles a leiomyoma, or a mass with focally infiltrative margins, so signal intensity is not a reliable indicator of malignancy (20). Furthermore, regarding high incidence of benign myoma in female population and the high price of MRI, casual screening for each case of a benign disease would be too expensive. Positron emission tomography/CT with fluorodeoxyglucose does not appear to be useful to distinguish between leiomyomas and uterine sarcomas. While the fluorodeoxyglucose uptake is generally high in sarcomas and low in leiomyomas, low malignancy or small sarcomas are less likely to be found with this type of imaging. Also, the uptake varies across individual tumours. Computed tomography (CT) does not reliably differentiate between leiomyomas and uterine sarcomas, however, CT is better imaging modality that MRI for lymph node imaging and staging. It is important to exclude other symptom-causing conditions in women with abnormal uterine bleeding, so endometrial biopsy would be a good choice for exclusion of endometrial hyperplasia or carcinoma. The endometrial sampling sensitivity is about 35% for diagnosis of leiomyosarcoma and about 25%, for endometrial stromal sarcoma, because more often the lesions are in the myometrium (21). Minimally invasive needle biopsy of the uterine mass guided by pelvic ultrasound or laparoscopy is not recommended. Limitations of this method are that the accurate diagnosis of sarcoma requires sampling of multiple sites and that the procedure may spill malignant cells within the peritoneal cavity. Rapid growth of a uterine mass during three months or the growth of a uterine mass in women in menopause should raise a suspicion of uterine sarcoma. Due to the absence of specific symptoms, the disease is very often diagnosed only postoperatively after examination of uterus specimen or when the disease is already widespread and secondary symptoms like dyspnea (due to distant metastasis of sarcoma in the lungs) appear. Histological criteria important to the diagnosis of the most common leiomyosarcoma are: nuclear atypia, increased cellularity, a high mitotic index, and geographic areas of coagulative necrosis. At least 2 criteria are needed to confirm leiomyosarcoma diagnosis.
Differential diagnosis
Today there are no validated clinical or radiological criteria that can accurately distinguish benign tumours from malignancies. It is difficult to rely on preoperative traditional imaging modalities, such as ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), to offer a reliable diagnosis. Moreover, only few cases could be diagnosed preoperatively from endometrial samplings, because most uterine sarcomas originate from the myometrium (12). Exacoustos et al. compared by ultrasound eight leiomyosarcomas, three smooth muscle uncertain malignant potential tumours, and 225 fibroids and found out that leiomyosarcomas were significantly bigger than other smooth muscle tumours. In seven out of eight cases their diameter was 8 cm or larger and better central vascularisation was observed (22). In another prospective study, 227 preoperative blood tests of patients with a smooth muscle tumour were analysed. An elevation of lactatdehidrogenasis (LDH) and its third form (LDH-3) was found in all ten women whose postoperative histological test confirmed leiomyosarcoma (23). Juang et al. analysed 42 cases of leiomyosarcoma and discovered that before operation the concentration of CA-125 was significantly higher in the leiomyosarcoma group compared with the leiomyoma group. They also noticed that CA-125 concentration did not differ between leiomyoma and early stage leiomyosarcoma (24). Cho et al. made a retrospective analysis and included 31 cases of sarcomas and 93 cases of leiomyomas into their study. They compared blood tests made one week before operation and discovered that the neutrophil count was significantly higher, whereas the lymphocyte count was significantly lower in the uterine sarcoma patients than in the myoma patients and therefore the neutrophil-lymphocyte ratio was significantly twice higher in the uterine sarcoma than in control patients – 3.9 ± 5.06 vs. 1.9 ± 1.16, respectively (25). In a recent study Kim et al. compared the neutrophil-lymphocyte ratio with serum CA‐125 as preoperative diagnostic markers for uterine sarcoma, and the neutrophil-lymphocyte ratio was found to be more useful as a diagnostic marker (26).
Treatment
Preoperative diagnosis is essential for characterisation of uterine tumours to determine the safest therapeutic strategy. Minimally invasive techniques, including laparoscopic intervention, morcellation, myomectomy, and uterine artery embolization have been developed for the treatment of uterine leiomyoma and are not appropriate for the treatment of uterine sarcoma to prevent dissemination (27, 28). In retrospective analysis it was found that women with LMS, who underwent morcellation, had an almost a three-times increased risk of a recurrent disease and worse overall survival regarding disease dissemination compared with the patients who underwent total abdominal hysterectomy and tumour consistency was not damaged (29, 30). That is why for the patients with the presumed diagnosis of uterine sarcoma it is important to perform CT, MRI or PET before operation in order to evaluate if the tumour is confined to the uterus or disease dissemination is seen. The standard treatment for uterine sarcoma is total abdominal hysterectomy (TAH) en bloc and bilateral salpingo-oophorectomy. The preservation of ovaries may be considered when the patient is a woman of reproductive age or for early-stage sarcoma. If uterine sarcoma is diagnosed after hysterectomy and especially when the uterus is fragmented by morcellation, or the cervix or ovaries remain, additional surgery should be considered after a pathologic review and imaging work-up for detecting extrauterine disease. If the uterine sarcoma is diagnosed through endometrium biopsy or myomectomy, additional surgery should be recommended to evaluate the disease extension and operability through an imaging work-up. Then standard surgical treatment is TAH with or without bilateral salpingo-oophorectomy and resection of metastatic lesions (28). For inoperable patients, pelvic radiotherapy with or without brachytherapy and/or chemotherapy is given and hormonal therapy can be considered. Lymphadenectomy is contraindicated for patients with uterine sarcoma (31).
The tumour stage is the most important prognostic factor that enables the evaluation of disease dissemination. Until 2009, the stages of uterine sarcomas were determined according to the endometrial carcinoma criteria established by the Federation of Gynaecology and Obstetrics (FIGO) and American Joint Committee on Cancer (AJCC). But these criteria were not accurate for uterine sarcomas, because both tumours – sarcoma and carcinoma – are of different origin. New criteria for uterine sarcoma staging were proposed by FIGO/AJCC in 2009. Currently this staging system is used for leiomyosarcoma, endometrial stromal sarcoma, and adenosarcoma, while carcinosarcomas are still staged by using endometrial carcinoma staging system (7).
Adjuvant therapy, recurrence, and metastatic disease treatment according to National Comprehensive Cancer Network (NCCN) guidelines (31)
Low-grade endometrial stromal sarcoma
When there are no symptoms after total hysterectomy with or without bilateral salpingo-oophorectomy in patients with stage I low-grade ESS, follow-up or hormonal therapy are recommended and no adjuvant therapy should be considered. For patients with stage II-IVA LGESS, treatment options are extra-beam radiotherapy (EBRT) and hormonal therapy. Palliative EBRT might be prescribed as additional treatment to hormonal therapy for patients with stage IVB disease.
High-grade endometrial stromal sarcoma, leiomyosarcoma and undifferentiated uterine sarcoma
The benefit of adjuvant therapy in disease treatment still remains unclear, but this therapy is usually given due to a high risk of a recurrent disease. In patients with stage I LMS, HGESS, and UUS after complete resection surgery, treatment options are follow-up, systemic therapy, and hormonal therapy. For patients with stage II-IVA LMS, HGESS, and UUS after complete resection surgery, adjuvant systemic therapy and/or EBRT should be considered. For patients with stage IVB or metastatic disease, systemic therapy or palliative EBRT should be considered.
Follow-up after treatment
Patient follow-up is recommended after uterine sarcoma treatment. Rectovaginal examination is recommended every 3–4 months in the first 2–3 years after treatment and later every 6–12 months (until the fifth year after treatment). During the visit, chest/abdomen/pelvic CT should be performed on the patient every 3–6 months in the first three years after treatment and later every 6–12 months for another two years. Patients also should be taught how to recognize possible symptoms of recurrence. Bleeding from the vagina, urine bladder or rectum, loss of appetite, weight loss, pain in the pelvis, abdomen, hips or in the back, shortness of breath, oedema in the legs – all these symptoms may possibly signal the recurrence, so the patient should urgently go to a doctor, without waiting for an arranged meeting.
Treatment of metastatic disease or disease recurrence
Management for locally recurred uterine sarcoma when disease recurs in vagina or in pelvis and imaging modalities show no distant metastatic disease, is determined by the presence or absence of previous RT. For patient, who had no previous RT it is recommended to perform a resection of recurred disease with or without tumour-directed intraoperative RT and preoprative systemic therapy. For residual disease EBRT along with (or without) brachytherapy and systemic therapy may be considered. Or instead of surgery, such patient may be treated with EBRT along with or without brachytherapy and systemic therapy. If the woman had a history of previous RT, then treatment options are: 1) resection of recurred disease with or without intraoperative RT and systemic therapy after surgery; 2) only a systemic therapy; or 3) re-irradiation and/or brachytherapy. Retrospective study data shows that for recurrence of endometrial stromal sarcoma, citoreductive surgery significantly improves survival (32).
For patients who have a resectable isolated metastasis, a surgical resection or other ablative therapy, like radiofrequency ablation or stereotactic body radiotherapy, should be performed; after the operation systemic therapy or EBRT should be considered. If isolated metastases are unresectable, then a systemic therapy should be given and considered regarding the operation if a response is seen, or for such patient local therapy like EBRT or ablative therapy should be performed.
If the patient has a disseminated disease, systemic therapy with or without pallative EBRT or best supportive care are recommended.
Treatment of carcinosarcoma
Despite the fact that the WHO classification of tumours of female reproductive organs classify carcinosarcoma as separate group of sarcomas, carcinosarcomas are included in the high-risk malignant epithelial tumours section according to the National Comprehensive Cancer Network (NCCN) uterine neoplasm treatment guidelines and the treatment is recommended to follow the guidelines for these malignant tumours. Primary treatment for these tumours includes total abdominal hysterectomy with bilateral salpingo-oophorectomy, peritoneal lavage for citology, omental and peritoneal biopsies; regarding maximal tumour debulking, pelvic and paraaortic lymphadenectomy should also be considered. Adjuvant therapy is individualised. For patients with stage IA disease without invasion to myometrium, there are three treatments options: (1) chemotherapy together with vaginal brachytherapy or without it, (2) observation if after hysterectomy there are no residual serous or clear cell carcinoma in the histological specimen, or (3) EBRT along with vaginal brachitherapy or alone. For all other patients with higher stages of the disease treatment with systemic therapy alone or along with tumour-directed RT is recommended.
Treatment of adenosarcoma
Primary treatment includes open abdominal hysterectomy, but it is not clear if bilateral adnexectomy and pelvic and paraaortic lymphadenectomy are necessary for this type of sarcoma. The use of adjuvant systemic therapy and radiotherapy in adenosarcoma also remains unclear, because there is a lack of studies showing the benefit of this treatment for this type of sarcoma. For residual disease or metastatic disease, when metastases are resectable, a surgical resection should be performed. Unresectable mestastases or isolated metastasis left after surgery should be treated with palliative RT. Apart from results of individual studies, there are no confirmed optimal systemic therapy regimens for the treatment of adenosarcoma recurrence. Having analysed 31 cases of adenosarcoma, Tanner et al. found that doxorubicin and ifosfamide therapy might be effective in the treatment of adenosarcoma and adenosarcoma with sarcomatous overgrowth (13, 33).
Prognosis
Leiomyosarcoma
The tumour stage is the most important prognostic factor that determines survival. Five-year survival rate for stages I and II fluctuates from 40% to 70%, the overall survival is 41%, and the recurrence rate is about 53–71% (14, 16, 19). The size is also an important prognostic factor for these soft tissue tumours. Tumours with a diameter less than 5 cm result in better survival rates (16).
Low-grade endometrial stromal sarcoma
The overall prognosis of this type of sarcoma is quite favourable. For stage I disease, 5-year survival rate is about 90%, and 50% for stages III–IV (14).
High-grade endometrial stromal sarcoma
In comparing low-grade ESS and high-grade ESS, in the case of high-grade ESS disease recurrence is usually observed earlier and more often. Mostly recurrence appears in less than one year, so there is a bigger risk of lethal prognosis (19). The survival rate prognosis is worse than in the case of low-grade ESS, but better than for undifferentiated uterine sarcoma (16).
Undifferentiated uterine sarcoma
In about 60% of cases, this type of sarcoma is found at stage III or stage IV. The survival prognosis is bad, because even when stage I disease is confirmed, the patient usually lives for only about two years (16, 19, 34). According to the data of the American Cancer Society, when this sarcoma is isolated only in the uterus, a 5-year survival rate is about 70%; when the disease is spread to the nearby tissues and lymph nodes – 43%, and when there are distant metastases, then the 5-year survival rate is only about 23% (35).
Adenosarcoma
When sarcomatous overgrowth or an invasion to the myometrium is observed, the risk of recurrence and metastatic disease increases. Uterine adenosarcomas recur in up to 30–40% of all cases and the most common place of recurrence is vagina (16, 33). For an early stage disease, 5-year survival is about 79% and for stage III – 48% (13, 14).
Carcinosarcoma
Progression of the disease is strongly associated with invasion to the myometrium. The most common place for metastases are the lungs and the peritoneum (36). The overall 5-year survival for patients with carcinosarcoma is 33–39% and 59–65% for stage I disease localised only in the uterus (37).
CONCLUSIONS
1. Uterine sarcomas are highly malignant tumours that develop from the smooth muscles and connective tissue elements of the uterus. They make up 1% of all malignant gynaecological tumours and about 3–7% of all malignant tumours of the uterus.
2. Uterine sarcomas are usually diagnosed in post-menopausal women aged from 50 to 70; the most common uterine sarcoma is leiomyosarcoma.
3. The disease has no specific symptoms, only a fibroid growing fastwithin a period of three months; an enlarging fibroid of the uterus in menopause may rise a suspicion of sarcoma.
4. Pre-operative diagnosis of uterine sarcoma remains difficult; imaging modalities cannot yet reliably distinguish benign myomas from malignant sarcomas.
5. It is important not to damage the integrity of the uterus during surgery and to morcellate the removable tumour in a special bag in order to prevent dissemination of the disease in the abdominal cavity.
6. The standard surgical treatment for uterine sarcoma is total abdominal hysterectomy (TAH) en bloc and bilateral salpingo-oophorectomy.
7. After surgical removal of uterine sarcoma, further investigation and treatment of the patient require a multidisciplinary care (consisting of an obstetrician gynaecologist, an oncologist gynaecologist, an oncologist chemotherapist, and a radiologist).
8. The low-grade endometrial stromal sarcoma has the best survival prognosis, while carcinosar-coma and undifferentiated uterine sarcoma have the lowest survival rates.
Diana Bužinskienė, Saulius Mikėnas, Gražina Drąsutienė, Matas Mongirdas
References
- Seagle B-LL, Sobecki-Rausch J, Strohl AE, Shilpi A, Grace A, Shahabi S. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017. April; 145(1): 61–70. [DOI] [PubMed] [Google Scholar]
- Hosh M, Antar S, Nazzal A, Warda M, Gibreel A, Refky B. Uterine sarcoma: analysis of 13,089 cases based on surveillance, epidemiology, and end results database. Int J Gynecol Cancer. 2016. July; 26(6): 1098–104. [DOI] [PubMed] [Google Scholar]
- Gockley AA, Rauh-Hain JA, del Carmen MG. Uterine leiomyosarcoma: a review article. Int J Gynecol Cancer. 2014. November; 24(9): 1538–42. [DOI] [PubMed] [Google Scholar]
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005. June; 48(2): 312–24. [DOI] [PubMed] [Google Scholar]
- Sivakumari S, Rajaraman R, Subbiah S. Uterine sarcoma: the Indian scenario. Indian J Surg Oncol [Internet]. 2015. September 14; 6(3): 232–6. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4856672/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trope CG, Abeler VM, Kristensen GB. Diagnosis and treatment of sarcoma of the uterus. A review. Acta Oncol. 2012. July; 51(6): 694–705. [DOI] [PubMed] [Google Scholar]
- Gupta M, Rajaram S. Uterine sarcomas: risk factors, clinical presentation, diagnosis, and staging BT – uterine cancer: diagnosis and treatment. In: Rajaram S, Chitrathara K, Maheshwari A. editors. New Delhi: Springer India; 2015. p. 339–50. Available from: 10.1007/978-81-322-18920_30 [DOI] [Google Scholar]
- Toro JR Travis LB Wu HJ Zhu K Fletcher CDM, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J cancer. 2006. December; 119(12): 2922–30. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Poen J, Tran LM, Fu YS, Heaps J, Parker RG. Postirradiation sarcoma of the gynecologic tract. A report of 13 cases and a discussion of the risk of radiation-induced gynecologic malignancies. Am J Clin Oncol. 1996. February; 19(1): 59–64. [DOI] [PubMed] [Google Scholar]
- Jaakkola S, Lyytinen HK, Pukkala E, Ylikorkala O. Use of estradiol-progestin therapy associates with increased risk for uterine sarcomas. Gynecol Oncol. 2011. August; 122(2): 260–3. [DOI] [PubMed] [Google Scholar]
- Wysowski DK, Honig SF, Beitz J. Uterine sarcoma associated with tamoxifen use. Vol. 346, The New England journal of medicine. United States; 2002. p. 1832–3. [DOI] [PubMed] [Google Scholar]
- Wu T-I, Yen T-C, Lai C-H. Clinical presentation and diagnosis of uterine sarcoma, including imaging. Best Pract Res Clin Obstet Gynaecol. 2011. December; 25(6): 681–9. [DOI] [PubMed] [Google Scholar]
- Denschlag D Thiel FC Ackermann S Harter P Juhasz-Boess I Mallmann P et al. . Sarcoma of the uterus. Guideline of the DGGG (S2k-Level, AWMF Registry No. 015/074, August 2015). Geburtshilfe Frauenheilkd [Internet]. 2015. October; 75(75): 1028–42. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4651298/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaska MJ, Haidopoulos D, Guyon F, Morice P, Zapardiel I, Kesic V. European Society of Gynecological Oncology statement on fibroid and uterine morcellation. Int J Gynecol Cancer. 2017. January; 27(1): 189–92. [DOI] [PubMed] [Google Scholar]
- Abeler VM, Royne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009. February; 54(3): 355–64. [DOI] [PubMed] [Google Scholar]
- Kurman RJ Carcangiu ML Herrington CS Young RH editors. . WHO classification of tumours of female reproductive organs. Fourth. Lyon; 2014. [Google Scholar]
- Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol. 1990. May; 14(5): 415–38. [DOI] [PubMed] [Google Scholar]
- D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010. January; 116(1): 131–9. [DOI] [PubMed] [Google Scholar]
- Jaime P, ’Nomonde M. Uterine sarcomas. Int J Gynecol Obstet [Internet]. 2015. September 30; 131(S2): S105–10. Available from: 10.1016/j.ijgo.2015.06.006 [DOI] [Google Scholar]
- Amant F, Coosemans A, Debiec-Rychter M, Timmerman D, Vergote I. Clinical management of uterine sarcomas. Lancet Oncol. 2009. December; 10(12): 1188–98. [DOI] [PubMed] [Google Scholar]
- Hinchcliff EM Esselen KM Watkins JC Oduyebo T Rauh-Hain JA Del Carmen MG et al. . The role of endometrial biopsy in the preoperative detection of uterine leiomyosarcoma. J Minim Invasive Gynecol. 2016; 23(4): 567–72. [DOI] [PubMed] [Google Scholar]
- Exacoustos C Romanini ME Amadio A Amoroso C Szabolcs B Zupi E et al. . Can gray-scale and color Doppler sonography differentiate between uterine leiomyosarcoma and leiomyoma? J Clin Ultrasound. 2007. October; 35(8): 449–57. [DOI] [PubMed] [Google Scholar]
- Brölmann H Tanos V Grimbizis G Ind T Philips K van den Bosch T et al. . Options on fibroid morcellation: a literature review. Gynecol Surg [Internet]. 2015. February 7; 12(1): 3–15. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4349949/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang CM, Yen MS, Horng HC, Twu NF, Yu HC, Hsu WL. Potential role of preoperative serum CA125 for the differential diagnosis between uterine leiomyoma and uterine leiomyosarcoma. Eur J Gynaecol Oncol. 2006; 27(4): 370–4. [PubMed] [Google Scholar]
- Cho H Kim K Kim Y-B No JH. . Differential diagnosis between uterine sarcoma and leiomyoma using preoperative clinical characteristics. J Obstet Gynaecol Res. 2016. March; 42(3): 313–8. [DOI] [PubMed] [Google Scholar]
- Kim HS Han KH Chung HH Kim JW Park NH Song YS et al. . Neutrophil to lymphocyte ratio for preoperative diagnosis of uterine sarcomas: A casematched comparison. Eur J Surg Oncol [Internet]. 2010. July 1; 36(7): 691–8. Available from: 10.1016/j.ejso.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Benson C, Miah AB. Uterine sarcoma – current perspectives. Int J Womens Health. 2017; 9: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-W Lee TS Hong DG No JH Park DC Bae JM et al. . Practice guidelines for management of uterine corpus cancer in Korea: a Korean Society of Gynecologic Oncology consensus statement. J Gynecol Oncol [Internet]. 2017. January 27; 28(1): e12 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5165063/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S Barysauskas C Serrano C Oduyebo T Rauh-Hain JA Del Carmen MG et al. . Retrospective cohort study evaluating the impact of intraperitoneal morcellation on outcomes of localized uterine leiomyosarcoma. Cancer. 2014. October; 120(20): 3154–8. [DOI] [PubMed] [Google Scholar]
- Park J-Y Park S-K Kim D-Y Kim J-H Kim Y-M Kim Y-T et al. . The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol Oncol. 2011. August; 122(2): 255–9. [DOI] [PubMed] [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms [Internet]. Version I. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx [Google Scholar]
- Yoon A Park J-Y Park J-Y Lee Y-Y Kim T-J Choi CH et al. . Prognostic factors and outcomes in endometrial stromal sarcoma with the 2009 FIGO staging system: a multicenter review of 114 cases. Gynecol Oncol. 2014. January; 132(1): 70–5. [DOI] [PubMed] [Google Scholar]
- Tanner EJ Toussaint T Leitao MMJ Hensley ML Soslow RA Gardner GJ et al. . Management of uterine adenosarcomas with and without sarcomatous overgrowth. Gynecol Oncol. 2013. April; 129(1): 140–4. [DOI] [PubMed] [Google Scholar]
- Tanner EJ, Garg K, Leitao MMJ, Soslow RA, Hensley ML. High grade undifferentiated uterine sarcoma: surgery, treatment, and survival outcomes. Gynecol Oncol. 2012. October; 127(1): 27–31. [DOI] [PubMed] [Google Scholar]
- The American Cancer Society: survival rates for uterine sarcoma by stage. October 12, 2017. [Google Scholar]
- Kanthan R, Senger J-L. Uterine carcinosarcomas (malignant mixed mullerian tumours): a review with special emphasis on the controversies in management. Obstet Gynecol Int. 2011; 2011: 470795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell LA, Blank SV, Duska LR. Uterine carcinosarcoma: A review of the literature. Gynecol Oncol. 2015. June; 137(3): 581–8. [DOI] [PubMed] [Google Scholar]