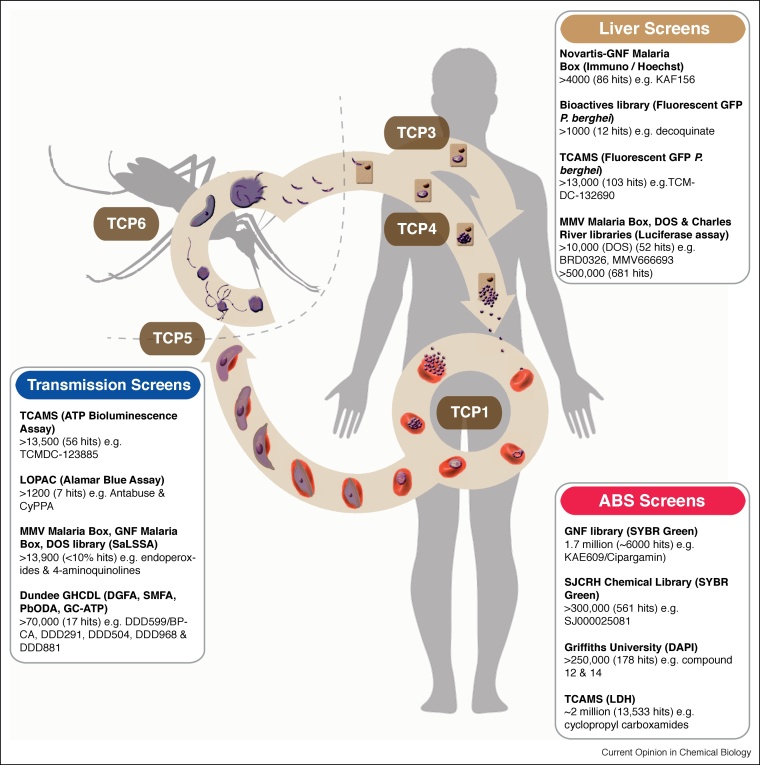
Figure 1.
The Plasmodium parasite lifecycle highlighting notable cell-based screens and Target Candidate Profiles (TCP) for developmental drugs.
The Plasmodium lifecycle occurs in stages between a mosquito vector and vertebrate host covering many different sites for drug intervention. Inoculation of motile sporozoites during the female Anopheles mosquito bloodmeal commences the asymptomatic liver stage. Exclusively to P. vivax and P. ovale, a proportion of liver-stage parasites form dormant hypnozoites (TCP3). Rupture of hepatic schizonts (TCP4) releases small merozoite forms that initiate the symptomatic stages (ABS, TCP1) made up of cycles of erythrocyte invasion, replication and release. A proportion of ABS parasites, rather than divide, commit to sexual differentiation to form the transmissible male and female gametocytes (TCP5), developing over 8–12 days (for P. falciparum), likely in the bone marrow, through morphologically distinct stages with sexual dimorphism most apparent at the mature stage V. Upon uptake to the mosquito during a bloodmeal, gametogenesis (formation of mature gametes), is induced rapidly (∼10–15 min). This follows environmental cues in the mosquito midgut, including a rise in pH, drop in temperature and the presence of xanthurenic acid, a mosquito-derived excretory product. Gametogenesis commences with the rounding up of both male and female gametocytes and their egress from the host erythrocyte. Male gamete formation, or exflagellation, is a remarkably rapid and tightly regulated process. The process includes three rounds of DNA replication alternating with endomitotic division, followed by the release of eight motile haploid male gametes. Fusion of male and female gametes ensues, leading to formation of a motile zygote that eventually colonizes the mosquito midgut, reseeding the vector for a new round of human infection [39]. Notable ABS cellular screens include those against the GNF Library; SJCRH (identifying hits with 50% inhibitory activity (IC50) of ≤2 μM); Griffiths University library (identifying hits for physicochemical and chemical diversity analysis) and TCAMS from GSK. Screens against the asymptomatic liver stages include screens of the Novartis-GNF Malaria Box (potent against ABS stages); bioactives library of commercially sourced compounds in clinical or pre-clinical development; TCAMS library (hits with dual blood and liver-stage activity) and the ultra-HTS of the MMV Malaria Box, DOS and most-recently Charles River libraries (hits with submicromolar exoerythrocytic stage activity). Transmission blocking screens to find drugs that block parasite transmission, compromising gametocyte or gamete viability, include those against the TCAMS library; LOPAC library using alamarBlue; MMV Malaria Box, GNF library and DOS library (using SaLSSA) and the Dundee GHCDL (using the DGFA).