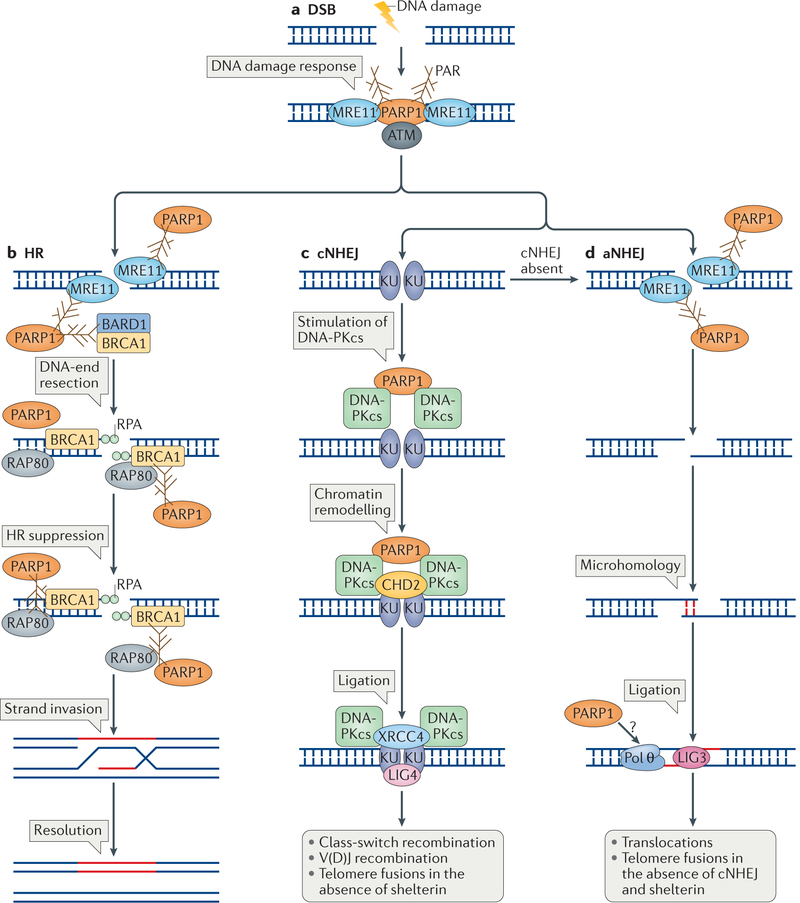
Figure 3 |. The roles of poly(ADP-ribose) polymerase 1 in detection and repair of DNA double-strand breaks.
a | Poly(ADP-ribose) polymerase 1 (PARP1) is required for the robust detection of DNA double-strand breaks (DSBs) and for the initial DNA damage response through its interaction with meiotic recombination 11 (MRE11) and the apical checkpoint kinase ataxia telangiectasia mutated (ATM). b | PARP1 has a role in DNA end resection during the homologous recombination (HR) process through the recruitment of MRE11 to DSBs, which is followed by binding of the single strand by replication protein A (RPA). This reaction also requires breast cancer type 1 susceptibility protein (BRCA1), and PARP1 may stimulate it by indirectly recruiting BRCA1 through its interaction with BRCA1-associated RING domain protein 1 (BARD1). PARP1 is also required at later steps to suppress HR, probably by limiting the extent of DNA end resection. Resection is limited by the stabilization of poly(ADP)ribosylated (PARylated) BRCA1, which is then bound by receptor-associated protein 80 (RAP80). The interaction with RAP80 stabilizes BRCA1 and suppresses HR, which results in limited strand invasion and in the subsequent repair of the DSB. c | PARP1 is also involved in DSB repair by stimulating classical non-homologous end joining (cNHEJ). When DSBs are channelled for repair by cNHEJ, they are bound by KU70–KU80 dimers, which activate DNA-dependent protein kinase catalytic subunit (DNA-PKcs). PARP1 interacts with DNA-PKcs and probably stimulates its activity without the requirement of KU proteins. PARP1 is also involved in chromatin remodelling during cNHEJ by facilitating the recruitment of chromodomain helicase DNA-binding protein 2 (CHD2), which then facilitates the recruitment of X-ray repair cross-complementing protein 4 (XRCC4) and DNA ligase 4 (LIG4) for DNA ligation. d | PARP1 has a role in alternative NHEJ (aNHEJ), which is active in the absence of cNHEJ. aNHEJ requires processing of the DNA ends by MRE11, which is recruited by PARP1. The resected ends are then joined though sequence microhomology and the gap is filled by DNA polymerase θ (Pol θ) and ligated by LIG3. Whether PARP1 is required for the recruitment of Pol θ is unknown (question mark).