Figure 3.
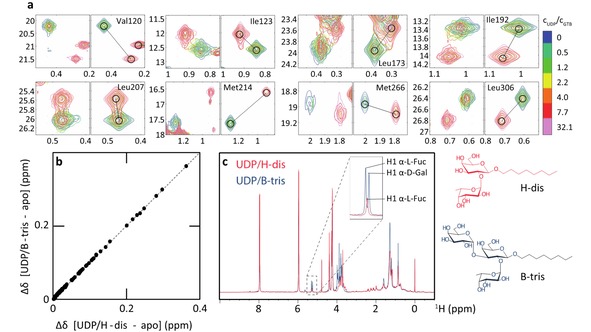
CSPs observed for a titration of GTB (193 μM), saturated with 1400 μM of the product of glycosyltransfer, B‐trisaccharide, (GTB:B‐Tri) with 6200 μM UDP (donor‐type ligand). a) Experimental (left panels) and fitted (right panels) line shapes of selected residues. The relative UDP concentrations are color‐coded. As in Figure 1c most resonances are in the limit of slow chemical exchange. However, higher UDP concentrations are needed to saturate the protein. b) Correlation of CSPs for GTB saturated with UDP and H‐disaccharide or B‐trisaccharide (CSPs are relative to the apo state). Each dot corresponds to a methyl group. An almost perfect linear correlation is obtained, indicating rather similar bound conformations. c) Proton NMR spectra of GTB in the presence of saturating amounts of UDP and H‐disaccharide (blue) or UDP and B‐trisaccharide (red), demonstrating integrity of the ligands.
