Fig. 5 |. Renal fibrosis in autosomal dominant polycystic kidney disease.
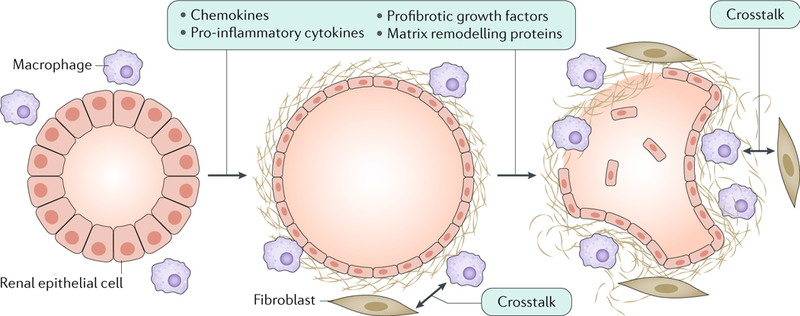
Altered expression of cystic proteins and dysregulated signalling in renal epithelial cells affect cell–extracellular matrix interactions and induce the production of chemokines (such as monocyte chemotactic protein 1 (MCP1), CC-chemokine ligand 6 (CCL6), CCL28, CXC-chemokine ligand 1 (CXCL1), CXCL8 and CX3C-chemokine ligand 1 (CX3CL1)), pro-inflammatory cytokines (such as tumour necrosis factor (TNF), IL-1, IL-2, IL-6, IL-8, macrophage colony-stimulating factor 1 (CSF1) and macrophage migration inhibitory factor (MIF)), profibrotic growth factors (such as transforming growth factor-β (TGFβ), TGFα, epidermal growth factor (EGF), fibroblast growth factors (FGFs) and platelet-derived growth factor (PDGF)) and proteins involved in matrix remodelling (such as matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs))299,300. These changes result in the accumulation of extracellular matrix and the recruitment of inflammatory cells, which is already observed in the early stages of the disease. In particular, M1 and M2 macrophages are important contributors to disease progression. Macrophages and interstitial fibroblasts also produce cytokines, and abnormal cytokine-mediated crosstalk between renal epithelial cells and inflammatory cells results in a positive feedback loop of increasing fibrosis.
