Abstract
Objective:
To evaluate the efficacy and safety of fluoroscopic self-expandable metallic stent (SEMS) placement for treating postoperative nonanastomotic strictures in the proximal small bowel.
Methods:
Data from 8 consecutive patients (mean age, 63.8 ± 6.9 years; 7 males and 1 female) who underwent 17 fluoroscopic SEMS placement procedures in total for treating postoperative nonanastomotic strictures in the proximal jejunum were retrospectively reviewed. The most recent surgery for all the patients was total gastrectomy with esophagojejunostomy. Strictures were located in the proximal jejunum in all patients. The mean length of the strictures was 5.8 ± 2.0 cm. Five patients with comorbidities were poor surgical candidates. Four patients underwent fluoroscopic balloon dilation, three of whom showed no resolution of obstructive symptoms and one demonstrated recurrence of symptoms.
Results:
Technical and clinical success was achieved in 100% (17/17) SEMS procedures. Complete resolution of obstructive symptoms and improvement in oral intake status occurred within 3 days after all procedures, rendering a clinical success rate of 100% (17/17). No complication occurred during or after the procedures. The median follow-up duration was 167 [interquartile range (IQR), 48–576] days. Stent malfunction occurred after 58.8% (10/17) of the procedures, including six occurrences of stent migration and four of benign tissue hyperplasia. Surgical removal of the migrated stents was performed in two patients. Recurrence of symptoms occurred after 64.7% (11/17) of the procedures. The median stent dwell and recurrence-free times were 32 (IQR, 20–193) and 68 (IQR, 38–513) days, respectively.
Conclusion:
Fluoroscopic SEMS placement may be effective and safe for treating postoperative nonanastomotic strictures, but stent malfunction and recurrence are major drawbacks.
Advances in knowledge:
SEMS placement is effective and relatively safe in patients with postoperative nonanastomotic strictures in the proximal small bowel. Patients section and counseling is highly encouraged.
Introduction
Postoperative nonanastomotic strictures are usually caused by adhesions and are the most common cause of small bowel obstruction.1 Although in the absence of peritonitis or ischemia, this condition can usually be managed nonoperatively, some patients require surgical intervention.2,3 Current clinical practice guidelines recommend that nonoperative management should not exceed 3–5 days because the possibility of spontaneous resolution is low after this period.2,3 However, in real practice, surgeons often extend nonoperative management to >5 days due to concerns of further adhesion formation from surgical exploration.4 Paradoxically, the incidence of small bowel obstruction increases with the number of operations performed for its management.3 In addition, surgery is associated with a very high morbidity rate and occasional mortality.5,6
Fluoroscopic balloon dilation (FBD) has been a valid treatment option for benign strictures of the esophagus and gastric outlet, and with developments in techniques and devices, it has also become a potential treatment option for lesions in the proximal small bowel (i.e. the duodenum and proximal jejunum).7–9 In a recent study involving 44 patients treated with FBD for postoperative nonanastomotic strictures in the proximal small bowel, 82% achieved clinical success (i.e. complete resolution of obstructive symptoms and resumption of oral intake of soft or solid food within 3 days of a technically successful FBD).10 Although this result is encouraging, a sizable portion (18%) of patients did not achieve clinical success. In addition, 27% of patients who achieved clinical success demonstrated recurrence.
Endoscopic and/or fluoroscopic self-expandable metallic stent (SEMS) placement is the first-line palliative option for malignant esophageal and gastroduodenal strictures.11,12 Although this procedure is associated with the risk of stent malfunctions (e.g. stent migration and tissue growth), it has also been used to treat refractory or recurrent benign strictures of the esophagus with satisfactory results.13–15 The use of SEMS placement for treating postoperative nonanastomotic strictures in the proximal small bowel in cases of clinical failure or recurrence after FBD seems reasonable, particularly for patients who are poor surgical candidates. In addition, it may be a valid option for patients with a limited life expectancy. To the best of our knowledge, no study on SEMS placement for treating postoperative nonanastomotic strictures in the proximal small bowel has been published. Therefore, the present study aimed to evaluate the efficacy and safety of fluoroscopic SEMS placement for treating postoperative nonanastomotic strictures in the proximal small bowel.
methods and materials
Study design
Our Institutional Review Board approved this retrospective study, and the requirement to obtain written informed consent was waived. The departmental electronic database was searched to identify eligible patients. The inclusion criterion was patients undergoing fluoroscopic SEMS placement for post-operative nonanastomotic strictures in the proximal small bowel at our institution between January 2000 and February 2016. The institutional indications for this procedure was more than 3 days of nonoperative management without resolution; contraindications were strangulation, peritonitis, incarcerated hernia, and intussusception. Diagnosis of post-operative nonanastomotic strictures in the proximal small bowel was made based on a history of abdominal surgery, clinical presentation (i.e. abdominal pain, distension, vomiting, and obstipation), radiological imaging studies (i.e. upper gastrointestinal series and CT), and endoscopic examination, when necessary. The exclusion criteria were patients with documented abdominal malignancy, inflammatory bowel disease, and abdominal irradiation history.
Patient population
In total, 8 patients (mean age, 63.8 ± 6.9 years; 7 males and 1 female) who underwent 17 fluoroscopic SEMS placement procedures in total were included. Three patients underwent multiple (range, 2–6) SEMS placement procedures because of recurrence. Before initial placement, one patient underwent multiple abdominal surgeries and had prior episodes of small bowel obstruction due to post-operative nonanastomotic strictures. No patient had undergone prior surgical treatment for small bowel obstruction. In all patients, the most recent abdominal surgery was total gastrectomy with esophagojejunostomy for gastric (N = 7) or metastatic (N = 1) cancer. The median interval from the most recent abdominal surgery to the initial episode of small bowel obstruction treated with SEMS placement was 167 [interquartile range (IQR), 97–823] days. All patients experienced vomiting with or without abdominal pain, distension, and obstipation. The stricture was located in the proximal jejunum in all patients. Two patients had complete obstruction and six had partial. All patients had a single stricture with a mean length of 5.8 ± 2.0 cm. The mean distance of the distal end of the stricture from the incisors was 46.7 ± 7.7 cm. Three patients were poor surgical candidates because of old age and/or comorbidities (i.e. hypertension, Type 2 diabetes mellitus, interstitial pulmonary fibrosis, lung cancer, and prostate cancer). Two patients preferred not to receive surgical management of the adhesions. Four patients had undergone FBD, three of whom experienced clinical failure and one demonstrated recurrence of obstructive symptoms. The remaining four patients had not undergone balloon dilation. One patient had interstitial pulmonary fibrosis and repeated episodes of severe pneumonia due to aspiration and therefore, the decision was made to perform SEMS placement rather than balloon dilation to prevent clinical failure. One patient had a limited life expectancy due to advanced lung cancer and therefore, the decision was made to palliate the patient by performing uncovered SEMS placement. In two patients, SEMS placement was favored over balloon dilation during the procedure because of a tight and/or torturous stricture.
Fluoroscopic SEMS placement and removal procedure
After fasting for at least 8 h, patients received topical pharyngeal anesthesia using an aerosol spray of lidocaine hydrochloride. A 0.035-inch 260-cm-long stiff-angled hydrophilic guidewire (Radiofocus M; Terumo, Tokyo, Japan) and a 5.4-Fr 160-cm-long multifunctional coil catheter (Song-Lim; S&G Biotech, Seongnam, Korea) were inserted through the mouth and navigated through the stricture under fluoroscopic guidance. The location and length of the stricture were identified by injecting a diluted water-soluble contrast medium (Ultravist 300; Schering Korea, Anseong, Korea) through the side-arm of the coil catheter. The exchange guidewire was then replaced with a 0.035-inch 260-cm-long super-stiff guidewire (Amplatz Super Stiff; Boston Scientific, Natick, MA), and the catheter was removed. A range of stents were used; a 16–20 mm diameter cm 6–16-long fully covered [EGIS Esophageal Stent (S&G Biotech) or Niti-S Esophageal (Taewoong, Ilsan, Korea)], partially covered (Hercules SP Pyloric; S&G Biotech), or uncovered (BONASTENT; Standard Sci-Tech, Seoul, Korea) SEMS was deployed over the super-stiff guidewire under continuous fluoroscopic monitoring, and an upper gastrointestinal series was performed to confirm good passage of the contrast medium through the SEMS. After the procedure, patients resumed oral liquid intake within 24 h and were not permitted any food until an upper gastrointestinal series after 1–3 days revealed complete stent expansion. When clinically necessary, the SEMS was fluoroscopically removed using a retrieval hook (S&G Biotech) as previously described.13
Follow-Up
All patients were evaluated for obstructive symptoms and oral intake capacity by clinical history-taking and daily examination during their hospital stay and at 1 month intervals on an outpatient basis after SEMS placement or whenever clinically necessary. An upper gastrointestinal series was performed at 1 month to exclude delayed complications. Further upper gastrointestinal series were only performed when clinically necessary.
Data collection and definitions
Data on demographics, clinical characteristics, technical success, procedural details, clinical success, complications, stent malfunctions, reinterventions, stent dwell time, and recurrence-free periods were obtained from the departmental electronic database. Technical success was defined as successful fluoroscopic SEMS placement at the desired anatomic location and good passage of contrast medium through the SEMS. Oral intake status was evaluated using dysphagia scores: 0 = normal swallowing, 1 = ability to swallow some solid diet, 2 = ability to swallow a soft diet, 3 = ability to swallow liquids only, and 4 = complete dysphagia.15 Clinical success was defined as complete resolution of obstructive symptoms and improvement in oral intake status by at least 1° within 3 days after SEMS placement. Recurrence was defined as symptoms recurrence due to stent malfunction. Complications were defined as bleeding, perforation, and/or aspiration. Stent malfunction was defined as stent migration, tissue growth, food impaction, and/or stent fracture.
Statistical analysis
The mean dysphagia score before and after SEMS placement was compared using the Student’s paired-samples t-test. A two-sided p-value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS v. 21 software (SPSS, Chicago, IL, USA).
Results
Technical success and procedural details
Technical success was achieved in 100% (17/17) of the procedures (Figure 1). Fully- or partially covered SEMSs were used in all but one procedure in which an uncovered SEMS was used because the patient had a limited life expectancy. The mean diameter and length of SEMSs were 16.6 ± 1.2 mm and 8.9 ± 2.0 cm, respectively. The median duration of the procedure (defined as the time interval from guidewire insertion to withdrawal) was 19 (IQR, 17–30) min.
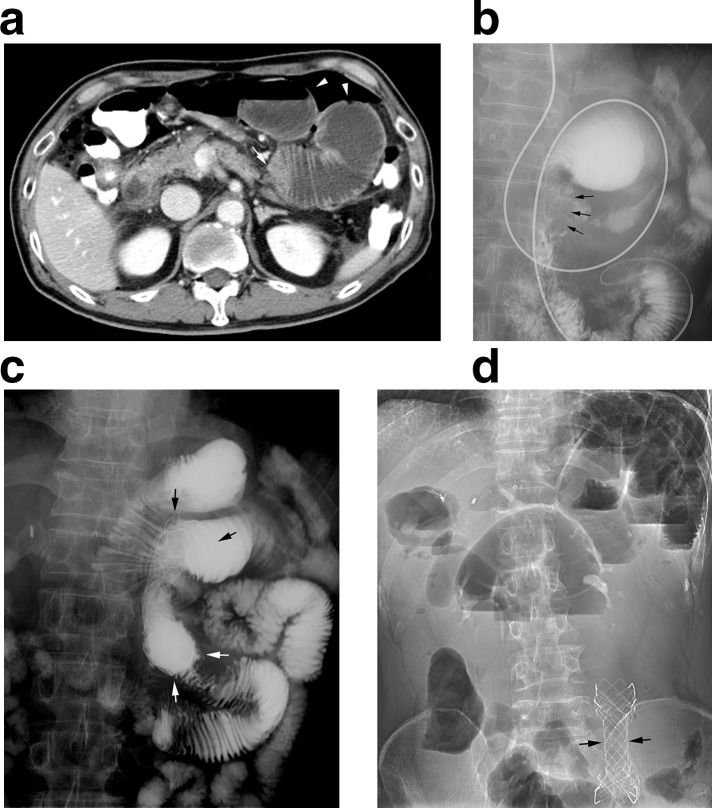
Figure 1.
A 63-year-old male (Patient no. 5) with a post-operative nonanastomotic stricture in the proximal small bowel 21 days after total gastrectomy with esophagojejunostomy. He demonstrated complete resolution of obstructive symptoms and resumed oral food intake within 3 days after placement of an 18 mm diameter 8-cm-long partially-covered SEMS (Hercules SP Pyloric; S&G Biotech). (a) CT image before SEMS placement showing a transition zone (arrow) at the proximal jejunum with a dilated bowel loop (arrowheads). (b) Radiograph before SEMS placement showing a 4-cm-long stricture (arrows) at the proximal jejunum distal to the anastomotic site. (c) Radiograph immediately after SEMS placement showing good passage of contrast medium through the SEMS (arrows). (d) Radiograph 79 days after SEMS placement showing the migrated SEMS in the distal small bowel (arrows). The patient underwent surgical removal of the migrated SEMS and remained recurrence-free for 1714 days. SEMS,self-expandable metallic stent.
Clinical success and complications
Clinical success was achieved in 100% (17/17) of the procedures. All patients had improved dysphagia score within 3 days after the procedure. The mean dysphagia score significantly improved from 3.1 ± 0.3 before SEMS placement to 1.8 ± 0.4 after SEMS placement (p<0.001). No complication occurred during or after the procedures. The clinical outcomes of SEMS placement in patients with postoperative nonanastomotic strictures in the proximal small bowel are shown in Table 1.
Table 1.
Clinical outcomes of SEMS placement in patients with post-operative nonanastomotic strictures in the proximal small bowel
| Patient no./Age (y)/Sex | Most recent abdominal Op | Stricture | Prior FBD | Comorbidities | SEMS | Dysphagia score | Complications/ Stent malfunction |
Reintervention | SEMS dwell time (d) |
Recur-ree time (d) | FU time (d) | |||||
| Time post -Op (d) |
Location | Length (cm) | Type | Length (cm) | Diameter (mm) | Before SEMS |
After SEMS | |||||||||
| 1/69/M | TG with EJ | 1332 | Prox Jej | 5 | + | HTN, T2DM | FC | 8 | 16 | 3 | 2 | -/Tissue hyperplasia | Required FSR | 32 | 38 | 167 |
| 1/69/M | TG with EJ | 1500 | Prox Jej | 7 | + | HTN, T2DM | PC | 8 | 16 | 3 | 2 | -/- | - | 35 | 35 | 35a |
| 2/56/M | TG with EJ | 823 | Prox Jej | 4 | - | IPF | PC | 5 | 18 | 3 | 2 | -/Stent migration | Required FSR | 24 | 59 | 59b |
| 3/72/M | TG with EJ | 97 | Prox Jej | 6 | - | Lung CA | UNC | 10 | 20 | 4 | 2 | -/- | - | 576 | 576 | 576c |
| 4/55/F | TG with EJ | 167 | Prox Jej | 3 | - | - | FC | 7 | 16 | 3 | 2 | -/Stent migration | Required SSR | 18 | 1778 | 1778 |
| 5/63/M | TG with EJ | 21 | Prox Jej | 4 | - | HTN | PC | 8 | 18 | 4 | 2 | -/Stent migration | Required SSR | 79 | 1714 | 1714 |
| 6/68/M | TG with EJ | 2070 | Prox Jej | 4 | + | Prostate CA | FC | 8 | 16 | 3 | 2 | -/- | Elective FSR | 31 | 68 | 72 |
| 6/68/M | TG with EJ | 2142 | Prox Jej | 4 | + | Prostate CA | FC | 8 | 16 | 3 | 1 | -/- | Elective FSR | 30 | 670 | 675 |
| 6/70/M | TG with EJ | 2817 | Prox Jej | 7 | + | Prostate CA | FC | 11 | 16 | 3 | 1 | -/Tissue hyperplasia | Required FSR | 225 | 225 | 231 |
| 6/71/M | TG with EJ | 3048 | Prox Jej | 7 | + | Prostate CA | FC | 11 | 16 | 3 | 1 | -/Tissue hyperplasia | Required FSR | 193 | 193 | 246 |
| 7/57/M | TG with EJ | 188 | Prox Jej | 3 | + | - | PC | 6 | 18 | 3 | 2 | -/Tissue hyperplasia | Required FSR | 237 | 237 | 733 |
| 8/70/M | TG with EJ | 141 | Prox Jej | 9 | + | - | FC | 13 | 16 | 3 | 2 | -/Stent migration | Required FSR | 4 | 21 | 33 |
| 8/71/M | TG with EJ | 174 | Prox Jej | 6 | + | - | FC | 10 | 16 | 3 | 2 | -/Stent migration | Required FSR | 4 | 10 | 14 |
| 8/71/M | TG with EJ | 188 | Prox Jej | 6 | + | - | FC | 10 | 16 | 3 | 2 | -/Stent migration | Required FSR | 5 | 58 | 64 |
| 8/71/M | TG with EJ | 252 | Prox Jej | 9 | + | - | FC | 10 | 16 | 3 | 2 | -/- | Elective FSR | 33 | 42 | 48 |
| 8/71/M | TG with EJ | 300 | Prox Jej | 9 | + | - | FC | 10 | 16 | 3 | 1 | -/- | Elective FSR | 369 | 513 | 520 |
| 8/72/M | TG with EJ | 820 | Prox Jej | 6 | + | - | FC | 8 | 16 | 3 | 2 | -/- | - | 20 | 20 | 20 |
CA, cancer; EJ, esophagojejunostomy; FBD, fluoroscopic balloon dilation; FC = fully-covered;FSP, fluoroscopic stent placement; FSR, fluoroscopic stent removal; FU, follow up; HTN, hypertension; IPF, interstitial pulmonary fibrosis; Jej, jejunum; OP, operation; PC, partially-covered; Prox, proximal; Recur, recurrence; SEMS, self-expandable metallic stent; SSR, surgical stent removal; T2DM, Type two diabetes mellitus; TG, total gastrectomy; UNC, uncovered.
Patient died due to myocardial infarction.
Patient died due to pneumonia.
Patient died due to recurrent lung cancer.
Stent malfunction and symptom recurrence
The median follow-up duration after SEMS placement was 167 (IQR, 48–576) days. No patient was lost to follow-up. Three patients died due to comorbidities (i.e. myocardial infarction, pneumonia, and recurrent lung cancer). Stent malfunction occurred after 58.8% (10/17) of the procedures over a median duration of 24 (IQR, 5–193) days, and stent migration occurred after 35.3% (6/17) of the procedures over a median duration of 5 (IQR, 4–24) days. Migrated SEMSs were fluoroscopically removed from four patients; the remaining two patients required surgical removal because the migrated SEMSs had become lodged in the distal small bowel. One of these patients initially refused surgical management. Benign tissue hyperplasia occurred after 23.5% (4/17) of the procedures over a median duration of 225 (IQR, 193–237) days. In all of these cases, SEMSs were fluoroscopically removed. The median stent dwell time after placement was 32 (IQR, 20–193) days. Elective fluoroscopic stent removal was performed for 23.5% (4/17) of the procedures after a median duration of 31 (IQR, 30–33) days. The median recurrence-free time after SEMS placement was 68 (IQR, 38–513) days. Recurrence of symptoms occurred in 64.7% (11/17) of the procedures. In two cases of recurrence, nonoperative management successfully resolved the patients’ symptoms, whereas the remaining nine cases of recurrence failed nonoperative management and underwent repeat fluoroscopic SEMS placement.
Discussion
The main advantage of temporary SEMS placement as compared with balloon dilation for treating benign esophageal strictures is the high rate of clinical success with refractory lesions.13,15 Therefore, SEMS placement is increasingly being used as an alternative to surgery for treating refractory benign esophageal strictures.16 For post-operative nonanastomotic strictures in the proximal small bowel, treatment with SEMS placement seems to confer the same advantage. SEMS can be placed and removed under endoscopic and/or fluoroscopic guidance.13–15,17 There are no consensus on the optimal guidance method for performing these procedures. However, it is well-perceived that the outcomes these procedures are the same, regardless of which guidance method is used.13,14 In the present study, all the SEMS were placed under fluoroscopic guidance with an initial clinical success with SEMS placement achieved after 100% (17/17) of the procedures. A recent study on 44 patients treated with FBD only achieved clinical success in 82% of patients.10 In addition, three patients in the current study experienced clinical failure with FBD. It should be emphasized, though, that the long-term clinical success is less favorable and patient selection and education is critical.
The recurrent nature of post-operative nonanastomotic strictures in the proximal small bowel represents a major problem.10,18 In the present study, recurrence of symptoms occurred after 64.7% (11/17) of the procedures. This rate is higher than that reported in a recent study on FBD (27%).10 However, the main advantage of SEMS placement for recurrent strictures is that the risk of symptom recurrence is low as long as the SEMS is temporarily in place.13,15 In addition, compared with balloon dilation, the effect of sustained dilation by SEMSs on strictures can lead to more durable patency even after stent removal.9 The median stent dwell time after the procedures was 32 (IQR, 20–193) days in the current study. This resulted in a median recurrence-free duration of 68 (IQR, 38–513) days, which is longer than that reported in a recent study on FBD [47 (IQR, 20–212) days], although four patients in the current study experienced clinical failure or recurrence with FBD.10 This suggested that SEMS placement is also useful for patients with recurrent lesions.
A major drawback of SEMS placement is stent malfunction.9,14 In the present study, covered SEMSs were used in the majority (16/17) of procedures. Fully covered SEMSs can prevent tissue ingrowth but are much more prone to migration than uncovered SEMSs,19,20 and partially covered SEMSs are less prone to migration than fully covered SEMSs but difficult to remove.21 In the current study, stent migration and benign tissue hyperplasia occurred after 35.3 and 23.5% of the procedures, respectively. However, these malfunctions were easily managed by fluoroscopic stent removal or, less frequently, by minor surgery when the migrated SEMS had become lodged in the distal small bowel. Uncovered SEMSs are prone to tissue ingrowth, which reduces stent migration and long-term patency and increases difficultly in removal.19,20 Therefore, these SEMSs are relatively contraindicated for benign strictures. However, uncovered SEMSs could be useful in patients with limited life expectancy. One patient in the present study had a limited life expectancy due to lung cancer; this patient underwent uncovered SEMS placement and remained well until death.
The present study has several limitations. First, this was a retrospective study and was therefore prone to selection bias. Second, the sample size of patients was small, which limits the strength of the results. Third, all SEMS placements were performed by experienced radiologists, and therefore, the results may not be generally applicable to others. Fourth, esophageal SEMSs were used in at the proximal jejunum as there are no specially designed stents available till now.
In conclusion, fluoroscopic SEMS placement appears to be effective for treating postoperative nonanastomotic strictures and useful for patients with refractory and recurrent lesions, particularly for those who have no alternatives like patients who are poor surgical candidates or have a limited life expectancy. However, it should be noted that stent malfunction and recurrence are major drawbacks of this technique. Patient selection should be very careful and the outcomes should be clearly discussed.
Footnotes
Acknowledgment: This study was funded by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Korea (Grant No.: HI15C0484 to HYS).
*These authors contributed equally to this work and are co-first authors.
Nader Bakheet and Jiaywei Tsauo have contributed equally to this study and should be considered as co-first authors.
Contributor Information
Nader Bakheet, Email: dr.nader.gamal@gmail.com.
Jiaywei Tsauo, Email: 80732059@qq.com.
Ho-Young Song, Email: hysong@amc.seoul.kr.
Kun Yung Kim, Email: kky2kkw@gmail.com.
Jung-Hoon Park, Email: jhparkz1125@gmail.com.
Zhe Wang, Email: scorpiowangzhe@163.com.
Min Tae Kim, Email: soir09@naver.com.
REFERENCES
- 1. Miller G , Boman J , Shrier I , Gordon PH . Etiology of small bowel obstruction . Am J Surg 2000. ; 180 : 33 – 6 . doi: 10.1016/S0002-9610(00)00407-4 [DOI] [PubMed] [Google Scholar]
- 2. Maung AA , Johnson DC , Piper GL , Barbosa RR , Rowell SE , Bokhari F , et al. . Evaluation and management of small-bowel obstruction: an eastern association for the surgery of trauma practice management guideline . J Trauma Acute Care Surg 2012. ; 73 ( 5 Suppl 4 ): S362 – 9 . doi: 10.1097/TA.0b013e31827019de [DOI] [PubMed] [Google Scholar]
- 3. Di Saverio S , Coccolini F , Galati M , Smerieri N , Biffl WL , Ansaloni L , et al. . Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2013 update of the evidence-based guidelines from the world society of emergency surgery ASBO Working Group . World J Emerg Surg 2013. ; 8 : 42 . doi: 10.1186/1749-7922-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schraufnagel D , Rajaee S , Millham FH . How many sunsets? timing of surgery in adhesive small bowel obstruction: a study of the nationwide inpatient sample . J Trauma Acute Care Surg 2013. ; 74 : 181 – 7 discussion 7-9 . doi: 10.1097/TA.0b013e31827891a1 [DOI] [PubMed] [Google Scholar]
- 5. Duron J-J , Silva NJ-D , du Montcel ST , Berger A , Muscari F , Hennet H , et al. . Adhesive postoperative small bowel obstruction: incidence and risk factors of recurrence after surgical treatment: a multicenter prospective study . Ann Surg 2006. ; 244 : 750 – 7 . doi: 10.1097/01.sla.0000225097.60142.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keenan JE , Turley RS , McCoy CC , Migaly J , Shapiro ML , Scarborough JE . Trials of nonoperative management exceeding 3 days are associated with increased morbidity in patients undergoing surgery for uncomplicated adhesive small bowel obstruction . J Trauma Acute Care Surg 2014. ; 76 : 1367 – 72 . doi: 10.1097/TA.0000000000000246 [DOI] [PubMed] [Google Scholar]
- 7. Kim JH , Shin JH , Song H-Y . Benign strictures of the esophagus and gastric outlet: Interventional management . Korean J Radiol 2010. ; 11 : 497 – 506 . doi: 10.3348/kjr.2010.11.5.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim JH , Shin JH , Di Z-H , Ko GY , Yoon H-K , Sung K-B , et al. . Benign duodenal strictures: treatment by means of fluoroscopically guided balloon dilation . J Vasc Interv Radiol 2005. ; 16 : 543 – 8 . doi: 10.1097/01.RVI.0000150033.13928.D4 [DOI] [PubMed] [Google Scholar]
- 9. Kim JH , Song H-Y , Park SW , Yoon CJ , Shin JH , Yook JH , et al. . Early symptomatic strictures after gastric surgery: palliation with balloon dilation and stent placement . J Vasc Interv Radiol 2008. ; 19 : 565 – 70 . doi: 10.1016/j.jvir.2007.11.015 [DOI] [PubMed] [Google Scholar]
- 10. Tsauo J , Kim KY , Song HY , et al. . Fluoroscopic balloon dilation for treating postoperative Nonanastomotic strictures in the proximal small bowel: a 15-year single-institution experience . J Vasc Interv Radiol 2017. ;. [DOI] [PubMed] [Google Scholar]
- 11. Lopera JE , Brazzini A , Gonzales A , Castaneda-Zuniga WR . Gastroduodenal stent placement: current status . Radiographics 2004. ; 24 : 1561 – 73 . doi: 10.1148/rg.246045033 [DOI] [PubMed] [Google Scholar]
- 12. Kim KY , Tsauo J , Song HY , Kim PH , Park JH . Self-Expandable metallic stent placement for the palliation of esophageal cancer . J Korean Med Sci 2017. ; 32 : 1062 – 71 . doi: 10.3346/jkms.2017.32.7.1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song HY , Jung HY , Park SI , Kim SB , Lee DH , Kang SG , et al. . Covered Retrievable expandable nitinol stents in patients with benign esophageal strictures: initial experience . Radiology 2000. ; 217 : 551 – 7 . doi: 10.1148/radiology.217.2.r00nv03551 [DOI] [PubMed] [Google Scholar]
- 14. Kim KY , Tsauo J , Song HY , et al. . Evaluation of a new esophageal stent for the treatment of malignant and benign esophageal strictures . Cardiovasc Intervent Radiol 2017. ;. [DOI] [PubMed] [Google Scholar]
- 15. Kim JH , Song H-Y , Choi EK , Kim KR , Shin JH , Lim J-O . Temporary metallic stent placement in the treatment of refractory benign esophageal strictures: results and factors associated with outcome in 55 patients . Eur Radiol 2009. ; 19 : 384 – 90 . doi: 10.1007/s00330-008-1151-2 [DOI] [PubMed] [Google Scholar]
- 16. Bakken JC . Wong Kee song LM, de Groen PC, baron th. Use of a fully covered self-expandable metal stent for the treatment of benign esophageal diseases . Gastrointest Endosc 2010. ; 72 : 712 – 20 . [DOI] [PubMed] [Google Scholar]
- 17. Yoon CJ , Shin JH , Song H-Y , Lim J-O , Yoon H-K , Sung K-B , et al. . Removal of Retrievable esophageal and gastrointestinal stents: experience in 113 patients . AJR Am J Roentgenol 2004. ; 183 : 1437 – 44 . doi: 10.2214/ajr.183.5.1831437 [DOI] [PubMed] [Google Scholar]
- 18. Irani S , Balmadrid B , Seven G , Ross A , Gan SI , Gluck M , et al. . Balloon dilation of benign small bowel strictures using double balloon enteroscopy: 5-year review from a single tertiary referral center . Gastrointestinal Intervention 2012. ; 1 : 74 – 8 . doi: 10.1016/j.gii.2012.08.007 [DOI] [Google Scholar]
- 19. Wei-Zhong Z . LT, sup, et al. stent placement in benign esophageal strictures . Gastrointestinal Intervention 2015. ; 4 : 69 – 75 . [Google Scholar]
- 20. Ji Hoon S , Jin-Hyoung K , Ho-Young S . Interventional management of benign strictures of the gastrointestinal tract from the stomach to the colon . Gastrointestinal Intervention 2013. ; 2 : 7 – 11 . [Google Scholar]
- 21. Jiaywei T , Jung-Hoon P , Ho-Young S , et al. . Development of gastroduodenal self-expandable metallic stents: 30 years of trial and error . Gastrointestinal Intervention 2016. ; 5 : 91 – 7 . [Google Scholar]