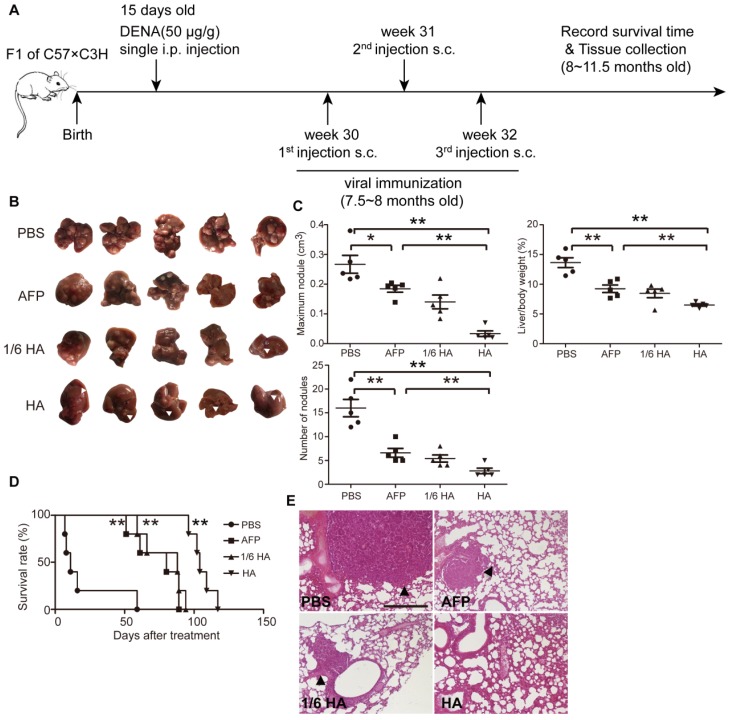
Figure 4.
Systemic assessment of lenti-HA in DENA-induced autochthonous HCC mice. Lenti-HA (3x107 or 5x106 copies) or lenti-AFP (3x107 copies) was subcutaneously administered into DENA-induced autochthonous HCC mice for 3 weeks at weekly interval. (A) Schematic diagram for the dosing regimen of lentiviral vaccines in DENA-induced autochthonous HCC mice. (B) Representative tumor images for treated autochthonous HCC mice. Arrowheads point to HCC nodules. (C) Analysis of tumor volume, weight and number of nodules from autochthonous HCC mice treated with lenti-HA or lenti-AFP (n=5, *P<0.05; **P<0.01). (D) Survival rate of autochthonous HCC mice treated with lenti-AFP or lenti-HA (n=5, **P<0.01). (E) Histological examination of pulmonary metastasis of HCC in lungs from autochthonous HCC mice treated with lenti-AFP or lenti-HA (scale bar=200 μm). Arrowheads point to HCC nodules.