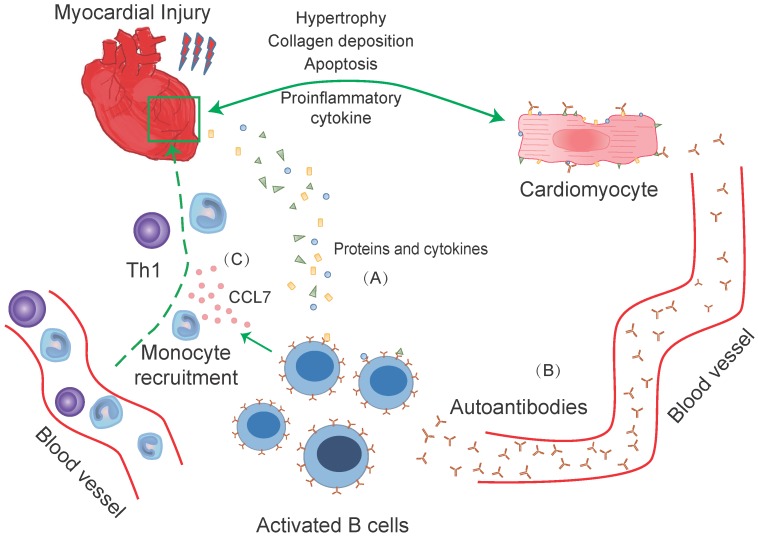
Figure 1.
B-cells response to heart injury in experimental and clinical studies. B-cells infiltrate into the myocardium after myocardial damage. Proteins and cytokines released from necrotic cardiomyocytes recognized by circular and cardiac infiltrated B-cells, causing B-cells activation. Then the activated B-cells induce direct cardiac injury via complement-mediated cytotoxicity and apoptotic signaling pathway (A). While, some activated circular B-cells transform into plasma and memory B-cells that produce autoantibodies. The recognition of autoantibodies and cardiomyocytes further cause deterioration of heart function. These include apoptosis of cardiomyocytes, increased collagen deposition, the release of pro-inflammatory cytokines, and rational hypertrophy (B). Meanwhile, activated B-cells, with the participation of Th1 cells, regulate the recruitment of Ly-6Chigh monocytes from the blood into injured myocardium through a CCL-7 dependent fashion. The supplement of cardiac monocytes aggravates the damage to heart function (C).