Table 1.
Reaction conditions optimization.[a]
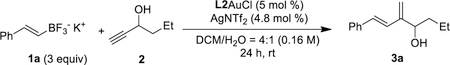 | ||
|---|---|---|
| Entry | Deviation from the optimized conditions | Yield(%)[b] |
| 1 | - | 77 |
| 2 | DCE/H2O (4:1) as reaction media | 69 |
| 3 | PhCF3/H2O (4:1) as reaction media | 67 |
| 4 | PhCH3/H2O (4:1) as reaction media | 0 |
| 5 | NaBARF instead of AgNTf2 as chloride abstractor | 70 |
| 6 | AgOTf instead of AgNTf2 as chloride abstractor | 70 |
| 7 | AgBF4 instead of AgNTf2 as chloride abstractor | 61 |
| 8 | 2 equiv of 1a; DCE/H2O (4:1) as reaction media | 56 |
| 9 | Ph3P, IPr, JohnPhos or MorDalPhos as Au ligand; 2 equiv of 1a; DCE/H2O (4:1) as reaction media | ≤6 |
| 10 | JohnPhos as Au ligand, 2 equiv of 1a; DCE/H2O (4:1) as reaction media; 5% Et3N | 16 |
| 11 | L3 as Au ligand; 2 equiv of 1a; DCE/H2O (4:1) as reaction media | 37 |
| 12 | L4 as Au ligand; 2 equiv of 1a; DCE/H2O (4:1) as reaction media | 18 |
| 13 | dry DCM used as solvent | 0 |
| 14 | (E)-styrylboronic acid instead of 1a | 35[c] |
| 15 | (Z)-1a instead | 0[d] |
0.1 mmol of 1-hexyn-3-ol (2) were used.
NMR yields.
27% 2 remained along with 22% of hydration product.
Mostly protodeboronation.
