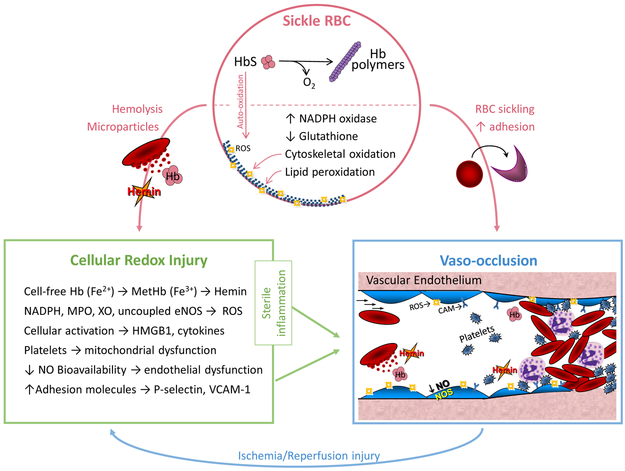
Figure 1: Redox Pathophysiology of Sickle Cell Disease (SCD).
Sickle RBC: A single point mutation in the beta globin (HBB) gene results in sickle hemoglobin (HbS), which reversibly polymerizes upon deoxygenation leading to red blood cell (RBC) sickling. Auto-oxidation of unstable HbS forms reactive oxygen species (ROS), upregulation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and depletion of glutathione, further contributing to RBC oxidative stress, cytoskeletal oxidation and lipid membrane peroxidation. Fragile sickle RBCs are prone to lysis, releasing intracellular components and microparticles into the circulation. Cellular Redox Injury: Cell-free Hb (Fe2+) can be oxidized to MetHb (Fe3+) by scavenging nitric oxide (NO) or reacting with H2O2 (Fenton reaction); once oxidized, hemin is readily released from MetHb. Increased circulating or cellular NADPH oxidase, myeloperoxidase (MPO), xanthine oxidase (XO) and uncoupled endothelial nitric oxide synthase (eNOS) also generate ROS in SCD. Immune cell and platelet activation release high mobility group box protein 1 (HMGB1) and cytokines. Hemin, ROS, HMGB1 and cytokines all promote sterile inflammation, endothelial dysfunction and increased expression of adhesion molecules, such as P-selectin and vascular cell adhesion molecule-1 (VCAM-1). Vaso-occlusion: RBC sickling and increased adhesion between the neutrophils, platelets, sickle RBCs and the vascular endothelium lead to stasis of blood flow, known as vaso-occlusion in SCD. Repeated episodes of vaso-occlusion produce ischemia/reperfusion injury that further contributes to cellular redox injury and sterile inflammation. Used with permission from Cheryl A. Hillery.