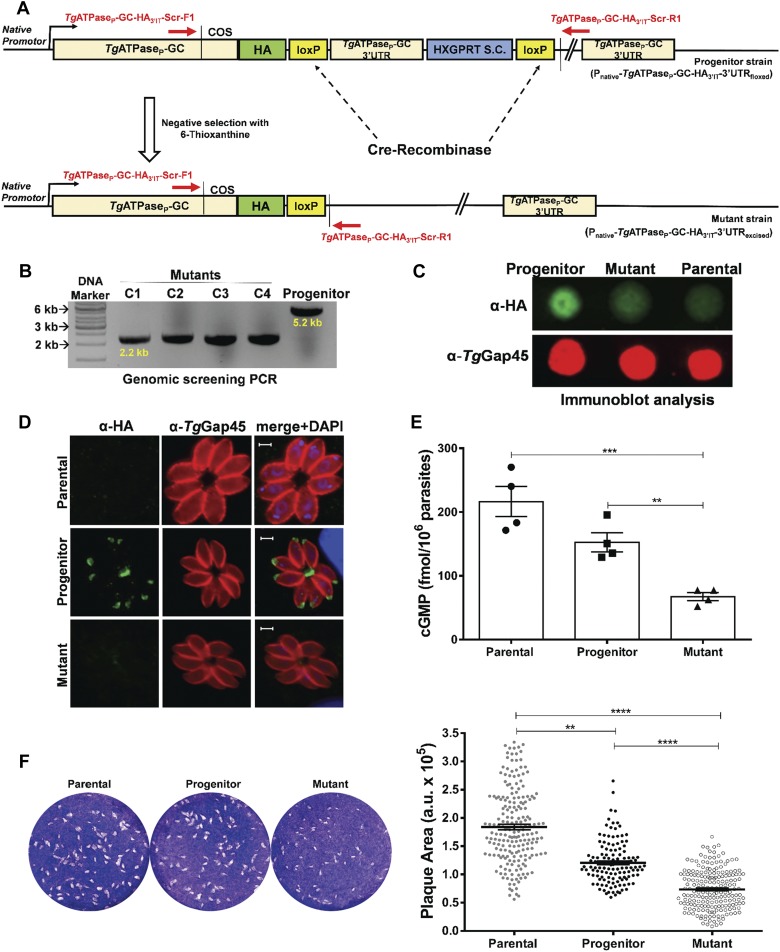
Figure 4. Cre recombinase–mediated down-regulation of cGMP synthesis impairs the lytic cycle of T. gondii.
(A) Schematics for making the parasite mutant (Pnative-TgATPaseP-GC-HA3’IT-3′UTRexcised). A vector expressing Cre recombinase was transfected into the progenitor strain (Pnative-TgATPaseP-GC-HA3’IT-3′UTRfloxed), in which 3′UTR of TgATPaseP-GC was flanked with Cre/loxP sites. Parasites transfected with a vector expressing Cre recombinase were selected for the loss of HXGPRT selection cassette (S.C.) using 6-thioxanthine. (B) Genomic screening of the TgATPaseP-GC mutant confirming Cre-mediated excision of 3′UTR and HXGPRT. Primers, indicated as red arrows in panel (A), were used to PCR-screen the gDNA isolated from four different mutant clones (C1–C4) along with the progenitor strain. (C) Immunoblot showing repression of TgATPaseP-GC-HA3’IT in parasites with excised 3′UTR with respect to the progenitor and parental (RHΔku80-hxgprt−) strains. Parasites (107) were subjected to the dot blot analysis using α-HA and α-TgGap45 (loading control) antibodies. (D) Immunostaining of the mutant (TgATPaseP-GC-HA3’IT-3′UTRexcised) and progenitor parasites revealing loss of HA signal in the former strain. Parental strain was used as a negative control for the background staining. Parasites were stained with α-HA and α-TgGap45 antibodies 24 h postinfection. Scale bars represent 2 μm. (E) Changes in the steady-state cGMP level of the mutant compared with the parental and progenitor strains. Fresh syringe-released parasites (5 × 106) were subjected to ELISA-based cGMP measurements (n = 4 assays). (F) Plaque assays using the TgATPaseP-GC mutant, progenitor, and parental strains. The dotted white areas and blue staining signify plaques and intact host-cell monolayers, respectively (left). The area of each plaque (arbitrary units, a. u.) embodies the growth fitness of indicated strains. 150–200 plaques of each strain were evaluated (right) from three assays. **P ≤ 0.01; ****P ≤ 0.0001.