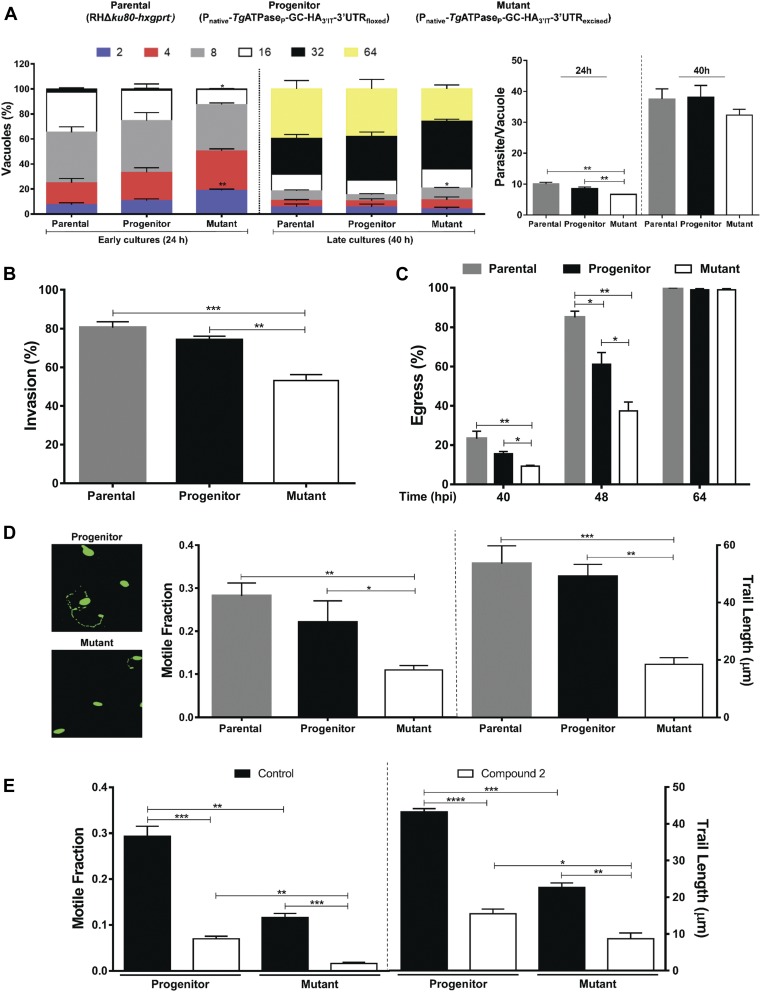
Figure 5. cGMP signaling governs the key events during the lytic cycle of T. gondii.
(A–D) In vitro phenotyping of the TgATPaseP-GC mutant, its progenitor, and parental strains. The intracellular replication (A), host-cell invasion (B), parasite egress (C), and gliding motility (D) were assessed using standard phenotyping methods. The progenitor and mutant strains were generated as shown in Figs 2A and 4A, respectively. The replication rates were analyzed 24 and 40 h postinfection by scoring the parasite numbers in a total of 500–600 vacuoles after staining with α-TgGap45 antibody (panel A, left) (n = 4 assays). The average parasite numbers per vacuole is also depicted (panel A, right). Invasion and egress rates were calculated by dual staining with α-TgGap45 and α-TgSag1 antibodies. In total, 1,000 parasites of each strain from four assays were examined to estimate the invasion efficiency. The natural egress of tachyzoites was measured after 40, 48, and 64 h by scoring 500–600 vacuoles of each strain (n = 3 assays). To estimate the gliding motility, fluorescent images stained with α-TgSag1 antibody were analyzed for the motile fraction (500 parasites of each strain), and 100–120 trail lengths per strain were measured (n = 3 assays). (E) Effect of PKG inhibitor compound 2 (2 μM) on the motility of TgATPaseP-GC mutant and its progenitor strain (500 parasites of each strain, n = 3 assays). A total of 100 trails in the progenitor, and 15 trails of the mutant (due to severe defect) were measured. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ****P ≤ 0.0001.