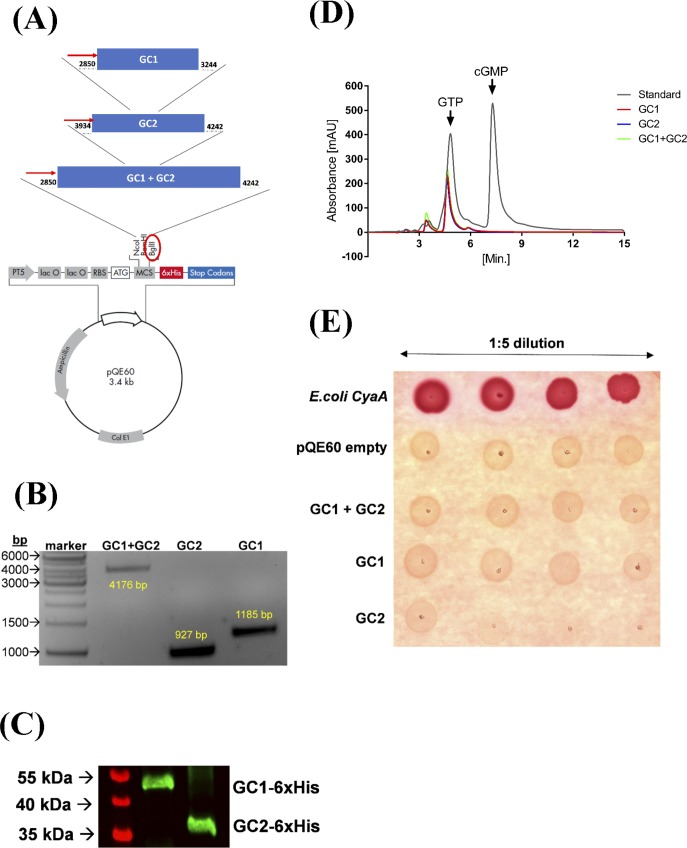
Figure S4. Expression of TgATPaseP-GC1 and GC2 domains in the M15 and BTH101 strains of E. coli.
(A) Scheme depicting the molecular cloning of GC1, GC2, and GC1+GC2 domains in the pQE60 expression vector. The open reading frames of indicated domains were amplified starting from the first upstream start codon (ATG) using the tachyzoite-derived RNA and ligated into BglII-digested pQE60 plasmid. Proteins were fused with a C-terminal 6xHis-tag for subsequent detection by immunoblot and purification by virtue of the Ni-NTA column. (B) PCR screening of transgenic M15 strains showing ORF-specific amplicons of GC1 (1,185 bp), GC2 (927 bp), and GC1+GC2 (4,176 bp). (C) Immunoblot of purified GC1-6xHis and GC2-6xHis proteins (5 μg) using the mouse α-His antibody. The protein bands of 47 and 38 kD correspond to GC1 and GC2 domains, respectively. Purification was performed under denaturing conditions from the M15 strain, GC1+GC2 could not be purified. (D) HPLC elution profile of cGMP and GTP after GC assay performed with bacterial lysates expressing GC1, GC2, or GC1+GC2 domains. Elution profiles of GTP and cGMP standards are also depicted (retention time for GTP ∼5 min, cGMP ∼7.5 min). Analyte elution was recorded by absorbance at 260 nm (mAU; milli-Absorbance Units). HPLC was performed on C18 reversed-phase column (3 μm particle size, 15 cm × 4.6 mm; SUPELCOSIL LC-18-T; Sigma-Aldrich) with a flow rate of 1 ml/min (sample injection volume, 20 μl). (E) Functional testing of GC1, GC2, and GC1+GC2 domains in the BTH101 strain, deficient in cAMP signaling. Transgenic bacterial strains expressing GC1, GC2, GC1+GC2, EcCyaA (positive control), or harboring the empty pQE60 vector (negative control) were grown in LB medium (OD600, 1.6; 37°C), and then dilution plated on MacConkey agar containing 1% maltose (carbon source) and 200 μM IPTG (inducer) followed by incubation at 30°C (∼32 h). The appearance of red colonies indicates cAMP-dependent catabolism of disaccharide after a functional expression of EcCyaA, but not others.