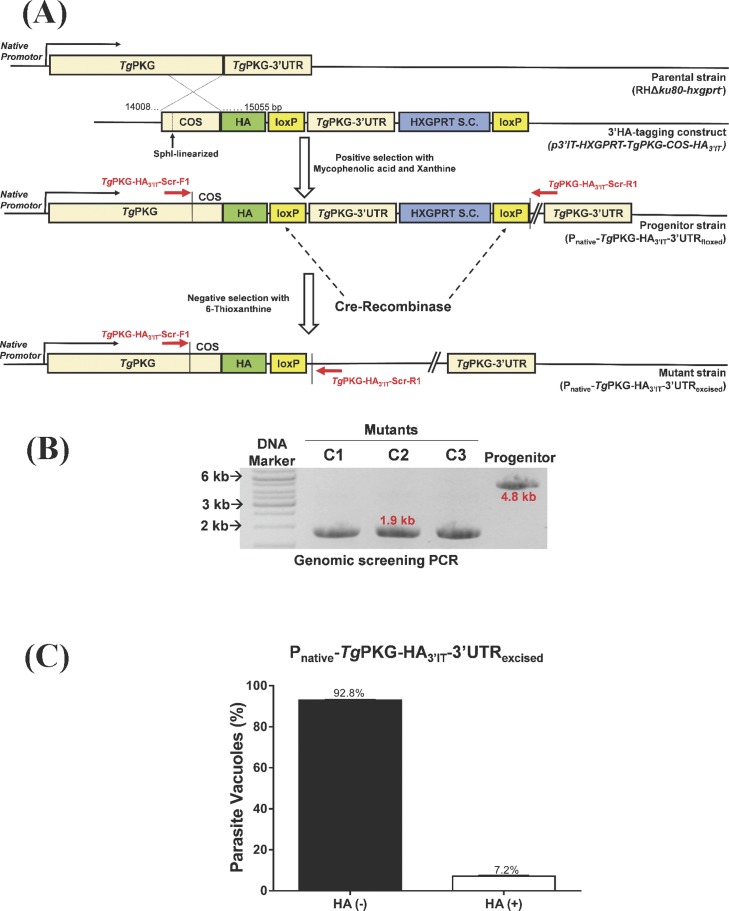
Figure S7. C-terminal epitope tagging and Cre recombinase–mediated knockdown of TgPKG in T. gondii.
(A) 3′-insertional tagging of the TgPKG gene with an HA epitope (step 1) and subsequent deletion of loxP-flanked (floxed) 3′UTR by Cre recombinase (step 2). The construct for 3′-insertional tagging (3′IT) via shown crossover sequence (COS) was transfected into the parental strain (RHΔku80-hxgprt−) and drug-selected for the HXGPRT selection cassette (S.C.). The eventual progenitor strain (Pnative-TgPKG-HA3’IT-3′UTRfloxed) expressed TgPKG-HA3’IT under its own regulatory elements. In the second step, the progenitor strain was transfected with a vector expressing Cre recombinase (pSag1-Cre) to cutoff the floxed 3′UTR and HXGPRT by negative selection, resulting in repression of TgPKG. (B) Genomic screening PCR validating the integrity of the TgPKG mutant generated by the excision of 3′UTR. Primers indicated as red-colored arrows in panel (A) were used to test gDNAs isolated from the mutant clones (C1–C3) and progenitor strain. The mutagenesis was further confirmed by sequencing of amplicons (1.9 kb). (C) Efficiency of 3′UTR excision in the Pnative-TgPKG-HA3’IT-3′UTRexcised mutant. The parasite vacuoles with or without HA signal were evaluated after staining of the mutant with α-HA and α-TgGap45, as shown in Fig 6A (350 vacuoles from n = 3 assays; mean with SEM).