Abstract
Background
Neuropathology has demonstrated a high rate of comorbid pathology in dementia due to Alzheimer’s disease (ADD). The most common major comorbidity is Lewy body disease (LBD), either as dementia with Lewy bodies (AD-DLB) or Alzheimer’s disease with Lewy bodies (AD-LB), the latter representing subjects with ADD and LBD not meeting neuropathological distribution and density thresholds for DLB. Although it has been established that ADD subjects with undifferentiated LBD have a more rapid cognitive decline than those with ADD alone, it is still unknown whether AD-LB subjects, who represent the majority of LBD and approximately one-third of all those with ADD, have a different clinical course.
Methods
Subjects with dementia included those with “pure” ADD (n = 137), AD-DLB (n = 64) and AD-LB (n = 114), all with two or more complete Mini Mental State Examinations (MMSE) and a full neuropathological examination.
Results
Linear mixed models assessing MMSE change showed that the AD-LB group had significantly greater decline compared to the ADD group (β = -0.69, 95% CI: -1.05, -0.33, p<0.001) while the AD-DLB group did not (β = -0.30, 95% CI: -0.73, 0.14, p = 0.18). Of those with AD-DLB and AD-LB, only 66% and 2.1%, respectively, had been diagnosed with LBD at any point during their clinical course. Compared with clinically-diagnosed AD-DLB subjects, those that were clinically undetected had significantly lower prevalences of parkinsonism (p = 0.046), visual hallucinations (p = 0.0008) and dream enactment behavior (0.013).
Conclusions
The probable cause of LBD clinical detection failure is the lack of a sufficient set of characteristic core clinical features. Core DLB clinical features were not more common in AD-LB as compared to ADD. Clinical identification of ADD with LBD would allow stratified analyses of ADD clinical trials, potentially improving the probability of trial success.
Introduction
Dementia due to AD (ADD) is frequently associated with comorbid pathologies that could affect clinical presentation, the rate of clinical progression, and response to investigational or approved treatments. For ADD, there are a host of prevalent co-existing vascular and molecular lesions [1–10].
The presence of comorbid and heterogeneous neuropathology would be a moot point if it had little or no clinical effect, or if it were only secondarily generated by the primary pathology. But if it resulted in an altered clinical disease progression rate and was not responsive to therapies directed at the “primary” pathology, it could have a significant impact on the response to such therapies. If these were both true, it is apparent that exclusion from ADD clinical trials of subjects harboring such neuropathological comorbidities would increase the observed effect size and hence clinical trial efficiency.
The most common comorbidity in ADD is Lewy body disease due to aggregation of α-synuclein. Approximately one-half or more of all subjects meeting clinicopathological diagnostic criteria for ADD also have α-synuclein pathology [9;11–13] of the Lewy body type (broadly termed “Lewy body disease”, LBD). Similarly, up to one-half of subjects with dementia and PD (PDD) [14–26] and three-quarters or more of those with DLB, have clinically significant AD pathology [27–30]. In the great majority of subjects with both ADD and LBD, this co-existence is recognized only at autopsy [31–33], currently preventing, except for the minority with clinically-typical DLB, exclusion of LBD subjects from ADD clinical trials. A number of autopsy-validated studies have indicated that cognitive decline is faster in elderly subjects dying with ADD, or any AD neuropathology, who also have unspecified LBD [3;34–37] but it is unclear whether or not these results may be driven primarily by DLB subjects with neuropathologically-severe LBD (neocortical stage diffuse Lewy body disease), as disease duration is reportedly shorter in this group [28;38]. The great majority of LBD in ADD subjects does not meet neuropathological diagnostic criteria for DLB, due to insufficient pathology density and brain regional distribution [39–41]. The presence of underlying α-synucleinopathy in these “AD-LB” cases is most often clinically silent [1] and they are usually diagnosed as probable ADD. We have previously reported thatscores for the Rey AuditoryVerbal Learning Test and Boston Naming Test are significantly worse for AD-LB than ADD, but that other neuropsychological variables are not significantly different [42]. Addtionally, depression and Trail-Making Test A scores correlated significantly with LBD pathology in AD-LB subjects. This study seeks to determine whether clinical disease progression differs in AD subjects with and without LBD. The prevalences of core DLB clinical features [40;41] were ascertained to help understand the failure to clinically identify many AD-DLB and AD-LB subjects.
Materials and methods
Subject selection
Subjects were selected by database searches of the Banner Sun Health Research Institute Arizona Study of Aging and Neurodegenerative Disorders (AZSAND)/Brain and Body Donation Program (www.brainandbodydonationprogram.org) [43], a subset of whom were also enrolled in the National Institute on Aging Arizona Alzheimer’s Disease Core Center. Search criteria specified that subjects died with dementia, two or more complete Mini Mental State Examinations (MMSE) and a full neuropathological examination after death. Of these, selected subjects met “intermediate” or “high” National Institute on Aging-Reagan Institute (NIA-RI) clinicopathological criteria [44] for ADD, with or without also meeting “intermediate” or “high” clinicopathological criteria for DLB (DLB III international consensus criteria)[40;41], or alternatively, for a group termed as AD-LB [39], also having pathologically-confirmed CNS LBD but not meeting DLB pathology distribution and density thresholds. For all subjects, other major neuropathological disorders were excluded, including cases clinicopathologically defined as vascular dementia, hippocampal sclerosis, frontotemporal lobar degeneration with TDP-43 proteinopathy, Pick’s disease, progressive supranuclear palsy, multiple system atrophy, corticobasal degeneration, Huntington’s disease, large acute cerebral infarcts, motor neuron disease and primary or metastatic malignant brain tumors. Selected subjects (Table 1) were divided into three groups: ADD (n = 137), AD-DLB (n = 64) and AD-LB (n = 114).
Table 1. Demographic and post-mortem characteristics of study subjects.
| AD | AD-DLB | AD-LB | p-value | |
|---|---|---|---|---|
| Number of Subjects | 137 | 64 | 114 | na |
| Gender (M/F) | 77/60 | 43/21 | 65/49 | 0.30 |
| Age at Death | 78.9±8.5 | 78.9±7.0 | 79.6±7.8 | 0.79 |
| Education (Yrs) | 14.8±2.6 | 14.6±3.1 | 14.1±2.6 | 0.16 |
| Cognitive Dysfunction (Yrs) | 7.9±4.0 | 8.0±4.0 | 8.9±3.7 | 0.10 |
| 1st to Last MMSE (yrs) | 4.1±2.9 | 4.4±3.1 | 4.4±2.4 | 0.56 |
| MMSE Assessments (n) | 4.4±2.1 range (2–11) |
4.9±2.4 range (2–11) |
4.8±2.3 range (2–13) |
0.10 |
| First MMSE Score | 22.1±6.3 | 23.5±5.9 | 22.9±5.6 | 0.26 |
| Last MMSE Score | 13.0±8.4 | 13.1±8.1 | 10.5±7.9 | 0.034 |
| Mean MMSE Score | 18.6±8.2 | 20.4±7.9 | 17.3±8.1 | < 0.0001 |
| Motor UPDRS | 17.9±17.1 (n = 56) |
29.7±21.7 (n = 28) |
16.2±17.4 (n = 34) |
0.008 |
| UPSIT | 24.6±8.3 (n = 28) |
13.6±4.4 (n = 20) |
22.2±7.0 (n = 15) |
< 0.0001 |
| Brain Weight (g) | 1119±137 | 1143±150 | 1133±156 | 0.44 |
| Total Plaque Score | 14.1±1.0 | 12.7±2.6 | 14.1±1.0 | < 0.0001 |
| Neuritic Plaque Density | 3.0 | 3.0 | 2.9 | 0.94 |
| Total Tangle Score | 12.4±3.4 | 10.1±3.9 | 13.5±2.6 | < 0.0001 |
| Braak NF Stage | 5 | 4 | 5 | < 0.0001 |
| Total LB Score | na | 29.1±7.4 | 9.5±6.9 | < 0.0001 |
| APOE ε4 Carrier y/n | 78/54 | 59/45 | 75/42 | 0.51 |
Means and standard deviations are shown; medians are shown for neuritic plaque density and Braak stage. Cognitive dysfunction duration is the number of years elapsed between first clinical appearance and death. Motor UPDRS scores for all groups were done off medications; the score shown is the final score prior to death. Group comparisons are by one-way analysis of variance or Kruskall-Wallis analysis of variance, as appropriate. Other comparisons are with chi-square tests or unpaired, 2-tailed Student t-tests. MMSE = Mini Mental State Examination; UPDRS = Unified Parkinson’s Disease Rating Scale; UPSIT = University of Pennsylvania Smell Identification Test; NF = neurofibrillary; LB = Lewy body; ApoE-E4 = apolipoprotein E, ε4 allele
Subject characterization
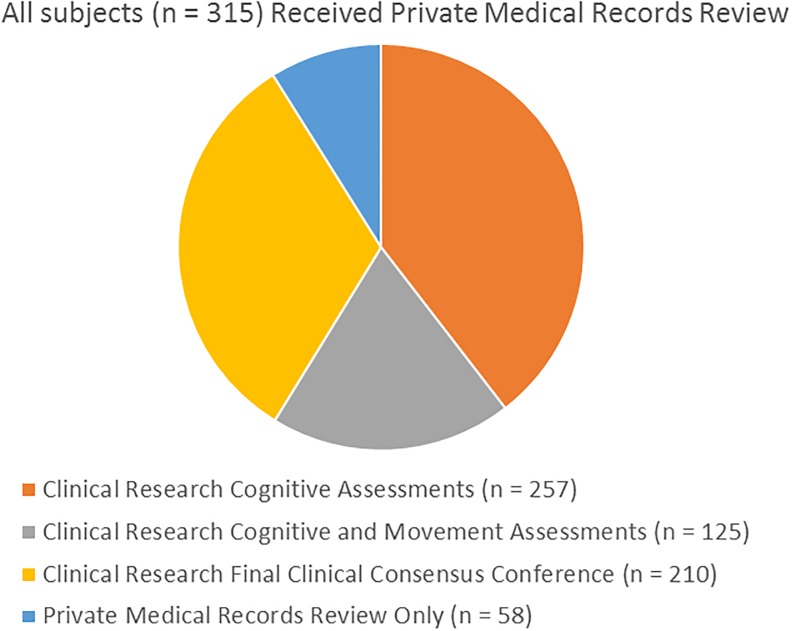
For all subjects, private medical records were reviewed, a comprehensive neuropathological examination was done after death and a final clinicopathological diagnosis was made by the neuropathologist and/or clinical research teams (Table 1 and Fig 1). Most subjects (n = 257) had serial standardized research cognitive evaluations and diagnostic consensus conferencesdone by teams of nurses, medical assistants, behavioral neurologists, neuropsychologists and psychometrists using standardized research-quality assessment batteries [43], including the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS). A subset of these (n = 210) received a final, postmortem clinical consensus cognitive diagnostic conference while still blinded to neuropathology findings. A further subset (n = 125; 58 AD, 31 AD-DLB and 36 AD-LB) additionally received serial assessments that included the Unified Parkinson’s Disease Rating Scale (UPRDS), administered by subspecialist movement disorders neurologists and 63 of these had one or more olfactory function assessments with the University of Pennsylvania Smell Identification Test (UPSIT). To estimate group prevalences of DLB core clinical features [40;41], the presence or absence of parkinsonism, visual hallucinations, Mayo Sleep Questionnaire findings [45–48] and/or clinical history consistent with REM sleep behavior disorder (RBD), as well as fluctuations in attention or cognition were recorded for each subject. The presence of PD, parkinsonism and visual hallucinations was additionally noted by standardized medical history questionnaires, and by review of private medical records.
Fig 1. Breakdown of clinical assessment types among the study sample.
All subjects received identical neuropathological examinations, including summary brain density measures for total amyloid plaques, neurofibrillary tangles, Lewy body pathology regional and summary density scoring, and staging using the Unified system [39], as well as assignment of CERAD neuritic plaque density and Braak neurofibrillary stage, as described previously [43].
All subjects gave written consent for study procedures and autopsy prior to enrollment and approval for the study and its consenting procedure was granted by the Western Institutional Review Board.
Statistical analysis
Demographic and post-mortem characteristics were analyzed using one-way analysis of variance (ANOVA), Kruskall-Wallis analysis of variance, Chi-square tests and t-tests as appropriate. Linear mixed-effects models were used to assess MMSE slope differences among the three groups. The first model included fixed effects for age (mean-centered), baseline MMSE score, diagnostic group, a visit age by group interaction term as covariates, and the random slopes for each subject. The second model added education, sex, and AD neuropathological severity (intermediate or high) according to the NIA-RI classification [44]. Fitted MMSE values from the second model were used to calculate annualized change for each of the groups. Kaplan-Meier survival curves for each diagnostic group (using time to 10-point MMSE decline as the outcome were compared using the log-rank test. The prevalence of core DLB features within and between the clinical groups were also reported and compared qualitatively.
Results
Clinical, demographic and post-mortem characteristics of the compared groups are shown in Table 1. No significant differences were noted for gender, age at death, proportion of subjects carrying the apolipoprotein E ε4 allele, education, duration of cognitive dysfunction until death, elapsed time between first and last MMSE, mean number of MMSE tests, duration between first assessment and death, or post-mortem interval. The groups were not significantly different on their first MMSE scores (p = 0.26) but differed on their mean (p < 0.0001) and final (p = 0.034) scores, with the AD-LB group having the lowest scores. Motor scores on the UPDRS and scores on the UPSIT differed significantly across groups (for both, p < 0.0001), driven by greater impairment in the AD-DLB group. Most subjects had a dementia diagnosis at study entry but approximately 20–35% were originally classified as cognitively normal or having mild cognitive impairment (MCI); the proportions were not significantly different between groups. Neuropathologically, subjects did not differ in their brain weights or neuritic plaque densities but were significantly different in total plaque scores, total tangle scores and Braak stage, with the AD-DLB group consistently having the lowest AD pathology scores. As expected by group definition, Lewy body pathology brain load was much higher for the AD-DLB group as compared to the AD-LB group. The lower AD-related pathology scores for the AD-DLB group were also expected due to classification rules for DLB that are more permissive when lower AD pathology densities are present [40].
The results from the first linear mixed-effects model showed that the AD-LB group had significantly greater decline compared to the ADD group (β = -0.69, 95% CI: -1.05, -0.33, p<0.001) while the AD-DLB group did not (β = -0.30, 95% CI: -0.73, 0.14, p = 0.18). Results from the second model with the additional covariates yielded nearly identical results: AD-LB (β = -0.68, 95% CI: -1.04, -0.32, p<0.001); AD-DLB (β = -0.29, 95% CI: -0.72, 0.14, p = 0.18). It was noted that in the second model AD pathology severity was significantly associated with MMSE decline (β = -8.00, 95% CI: -12.54, -3.45, p<0.001). The MMSE slopes for the AD-DLB and AD-LB groups were not significantly different between the adjusted and unadjusted models (both comparisons were, p = 0.17). Linear mixed model-derived annualized MMSE changes for each group are shown in Table 2. Since the effect AD pathology severity was quite large in the second model, we ran an additional analysis where only AD-DLB subjects with a NIA-RI classification of ‘High’ were included. This resulted in the three groups having statistically similar plaque and tangle burdens, however the MMSE trajectories followed the same pattern as those in the previous linear mixed models: AD-LB (β = -0.68, 95% CI: -1.05, -0.32, p<0.001); AD-DLB (β = -0.39, 95% CI: -0.97, 0.19, p = 0.19).
Table 2. Annualized MMSE change for ADD, AD-DLB, and AD-LB groups.
| MMSE Annualized Change | |
|---|---|
| ADD | -1.48±0.70 (-1.60, -1.35) |
| AD-DLB | -1.77±0.86 (-1.99, -1.55) |
| AD-LB | -2.16±0.76 (-2.30, -2.01) |
Mean ± standard deviation (95% Confidence Interval)
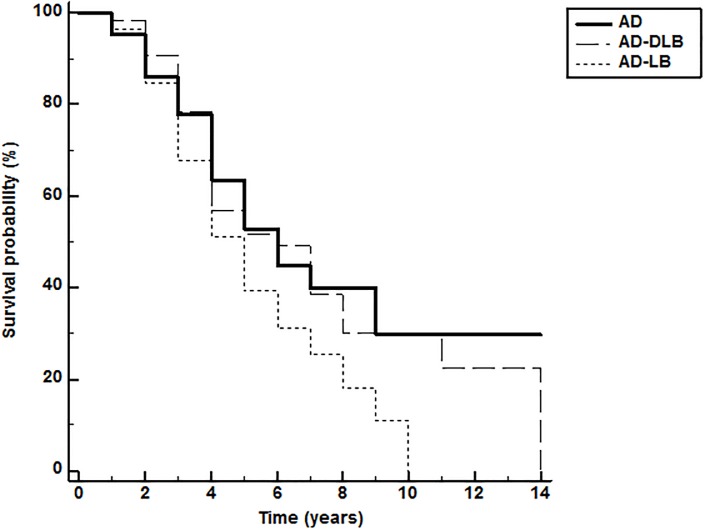
To further compare rate of cognitive decline between the diagnostic groups, we constructed Kaplan-Meier curves (Fig 2) for survival to a 10-point MMSE decline. For the AD, AD-DLB and AD-LB groups this was 7.49 years (95% CI: 6.36, 8.62), 7.21 years (95% CI: 5.78, 8.64) and 5.25 years (95% CI: 4.64, 5.86), respectively. The log-rank test for the survival curve differences was statistically significant (p = 0.03; Fig 2); the AD-LB had a significantly faster time to a 10-point MMSE decline relative to the AD group (HR = 1.51; 95% CI: 1.04, 2.19). All other groupwise comparisons were not statistically significant.
Fig 2. Kaplan-Meier curve for time to 10-point MMSE decline.
The log-rank test for the survival curve differences was statistically significant (p = 0.03; Figure 2); the AD-LB had a significantly faster time to a 10-point MMSE decline relative to the AD group (HR = 1.51; 95% CI: 1.04, 2.19). All other groupwise comparisons were not statistically significant.
Lewy body disease diagnosed at autopsy was often unrecognized during life, even when examiners were specialized behavioral neurologists, neuropsychologists and psychometrists using standardized research-quality assessments, including the NACC UDS, UPDRS and Mayo Sleep Questionnaire. Of those meeting both NIA-RI intermediate or high neuropathological criteria for AD as well as DLB III [40] neuropathological criteria for intermediate or high likelihood for DLB, and that had a final postmortem (blinded to neuropathology) clinical diagnostic conference from the specialist research team, 21/32 (66%) had been clinically diagnosed at least once (any Lewy body related clinical diagnosis at any time during their clinical course was accepted) with DLB, “Lewy body dementia” or “Lewy body variant of Alzheimer’s disease” by either the research teams of specialized neurologists and neuropsychologists or by private medical practitioners. However, only 15/32 (46.9%) of these were diagnosed as DLB at the specialist research team’s final postmortem (blinded to neuropathology results) clinical diagnostic conference. For those with AD-LB, the percentage diagnosed by the research teams at any time with a Lewy body related diagnosis was 2/93 (2.1%). In comparison, for those with AD and no postmortem evidence of Lewy body disease, 4/85 (1.6%) had been diagnosed at least once as having a Lewy body related diagnosis.
To determine why the diagnosis of LBD was being missed relatively frequently by the clinical research teams, we examined the group prevalences of core DLB clinical features, including parkinsonism, visual hallucinations, cognitive fluctuations and history consistent with RBD and/or dream enactment behavior.
Of the neuropathologically-defined AD-DLB cases evaluated at least once by the specialized clinical research teams, 58% had parkinsonism, 47% had hallucinations, 33% had cognitive fluctuations and 18% had a history of RBD and/or dream enactment behavior. Of the AD-LB subjects that had at least one evaluation by the research teams, the core features were much less common: 30% had parkinsonism, 11% had hallucinations, 13% had fluctuations and 5% had RBD and/or dream enactment behavior. The difference in prevalences were all significantly greater for AD-DLB versus the AD-LB and ADD groups (p < 0.05) (Table 3). Emphasizing the relative infrequency of core features in the AD-LB group, their prevalences did not significantly differ from the ADD group. The presence of all core features, except cognitive fluctuation, were significantly more common in the clinically-recognized AD-DLB subjects as compared to those meeting DLB neuropathological criteria (DLB Consortium intermediate or high) but who were unsuspected as such during life.
Table 3. Prevalence of DLB core features in AD-DLB subjects with and without a clinical DLB diagnosis.
| parkinsonism* | hallucinations* | fluctuations | RBD/DEB* | |
|---|---|---|---|---|
| DLB (clin/path) |
18/24 (75%) |
16/24 (67%) |
9/24 (38%) |
8/24 (33%) |
| DLB (path) |
14/31 (45%) |
10/31 (32%) |
9/31 (29%) |
2/31 (6%) |
* = p < 0.05;
Only data from subjects seen at least once by clinical research teams is considered. Clin/path = subjects diagnosed as DLB both clinically and neuropathologically. Path = subjects without a clinical diagnosis of DLB but neuropathologically meeting DLB III intermediate or high criteria. RBD/DEB = clinical diagnosis of REM sleep behavior disorder or presence of dream enactment behavior.
Discussion
The results confirm prior findings by others that cognitive decline is faster in subjects with autopsy-confirmed ADD if they also have co-existing LBD neuropathology [3;28;34–38;49]. A new finding is that there is an increased rate of decline for AD-LB subjects relative to ADD subjects, with AD-DLB showing a rate of MMSE decline that is in between the ADD and AD-LB groups. A large fraction of AD-DLB cases, and virtually all the AD-LB cases, were unrecognized as having LBD during life, even though they had at least one and in many cases annual evaluations by specialized research teams including both behavioral and movement disorders subspecialist neurologists. As AD-LB is much more common than AD-DLB (33% and 16% of all ADD cases, respectively, our unpublished data), this means that approximately 40% or more of ADD subjects have co-existing, clinically-silent LBD that accelerates their disease progression. Aside from complicating clinical trial analyses using rate of cognitive decline as a marker of therapeutic response, it is still unknown whether or not therapy directed at AD molecular targets will have any effect on LBD. It is possible that an agent might slow or halt the growth of AD pathology but be clinically ineffective due to the unobstructed advance of LBD.
It is surprising that LBD conferred a faster rate of cognitive deterioration regardless of the severity and extent of LBD pathology. The difference from ADD was in fact restricted to the AD-LB group, despite the greater LBD pathology densities and regional brain distribution in AD-DLB. In AD-DLB subjects, LBD pathology has most often spread to the neocortical stage, while in AD-LB it is most often restricted to a limbic-predominant stage [39], and the density of LBD pathology is almost always greater in AD-DLB, in every involved brain region. As the difference in rate of decline between AD-LB and ADD is maintained even after adjustment for level of AD neuropathology, it seems likely that AD-LB is an inherently more aggressive disease than ADD, while AD-DLB is similar to ADD. The reason(s) for this, however, are unclear at this time, although it might be speculated that cortical LBD are somehow protective with respect to AD-type neurodegeneration, or that qualitative, rather than quantitative, group pathology differences may be responsible, such as structural “strain” differences in α-synuclein, Aβ or tau aggregates.
It has been reported that AD-DLB may be a more malignant condition than ADD, based on a reportedly more rapid disease course from first presentation to nursing home placement or death [50;51]; conversely, the rate of cognitive decline has most often been found to be similar in the two conditions [50–52]. Others have shown that survival time, in those with clinically probable, neuropathologically-confirmed DLB, is significantly shorter with diffuse neocortical LBD as compared with LBD restricted to the limbic regions [38] and that this is at least partially related to greater AD pathology, the presence of lacunar infarcts and parkinsonism; this cited study did not compare survival between ADD and AD-DLB. In the current study, age at death and survival did not differ between ADD and AD-DLB, even when AD-DLB cases were restricted to those with neocortical stage (results for survival and for the separated neocortical group not shown). Differences between our study and that cited above [38], aside from lack of an ADD comparison group in the latter, include our case selection, based on neuropathologically-defined DLB, rather than a combined clinical and neuropathological definition, as well as our exclusion of cases meeting vascular dementia neuropathological criteria, resulting in an almost complete lack of lacunar infarcts in our AD-DLB group (only 3 cases had any lacunar infarcts). One other study [28], based on neuropathologically-defined DLB, confirmed a shorter disease duration to death in neocortical stage LBD as compared to limbic stage, but did not include an ADD comparison group.
A limitation to these conclusions is that the MMSE is a single, relatively simple test and comprehensive neuropsychological test comparisons would be more informative. Unfortunately, more sophisticated neuropsychological tests often cannot be performed in subjects with advanced dementia and so the sample size is much reduced. In a reduced subject set with full neuropsychological test battery results, we previously reported that scores for the Rey Auditory Verbal Learning Test and Boston Naming Test are significantly worse for AD-LB than ADD, but that other neuropsychological variables are not significantly different [42]. There is more brainstem and subcortical damage in the AD-DLB group than in the AD-LB group and it is possible that selected neuropsychological tests that are based on brainstem function, such as some tests of working memory [53], might show a greater rate of decline in the AD-DLB group.
Whether or not LBD initiation or spread is independent, in ADD subjects, of the AD neuropathology, is not yet established. While experimental models have shown potential synergistic or overlapping molecular mechanisms generating AD and LBD pathology [54–57], it is also apparent that LBD is commonly present in subjects where AD pathology is clearly primary, such as in autosomal dominant mutations of PS1 and APP [57–62], or even in other cerebral amyloidoses [63]. It is possible, then, that much of the LBD in AD subjects is produced as a secondary event and thus effective anti-amyloid or anti-tau agents might also be effective against LBD. To investigate the possibility of differential effects of therapeutic agents on AD and LBD pathology, it will be necessary to first acquire much more sensitive and specific LBD clinical diagnostic methods.
Clinically-typical DLB is readily and accurately identified when the core clinical features are present but in this set of subjects these were only 31% of all those with ADD and LBD. The cognitive presentation may be helpful, as initial impairment in attention, executive function and/or visuospatial function may be more common than in AD without LBD [64]. The presence of REM sleep behavior disorder (RBD) is a strong predictor of underlying LBD [65] but this relationship is weaker for the relatively low LBD pathology loads typical of AD-LB [45;66]. In this study, as in others [67;68] decreased olfaction is strongly associated with LBD. The association was restricted to the AD-DLB group however, as UPSIT scores for the AD-LB and pure AD groups were not significantly different. Although the sample size for all groups is relatively small, these results suggest that hyposmia or anosmia may be a key diagnostic feature of DLB.
This study found that undetected LBD in ADD individuals is associated with faster cognitive decline relative to ADD individuals without LBD. The absence of reliable fluid and imaging markers for LBD means that its contribution to cognitive decline in observational and intervention studies of ADD is unknown, which could lead to biased estimates of cognitive trajectories and treatment effects. Better clinical diagnostic methods for LBD are critically needed, as the identification of AD-LB subjects entered into ADD clinical trials would enable their exclusion or a stratified analysis that would potentially increase the probability of trial success.
Data Availability
Data cannot be shared publicly because of identifying information contained in the dataset. Data are available from Banner Sun Health Research Institute (www.brainandbodydonationprogram.org) for researchers who meet the criteria for access to confidential data.
Funding Statement
The Brain and Body Donation Program has been supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. Dr. Kewei Chen is employed by Shanghai Green Valley Pharmaceutical. Shanghai Green Valley Pharmaceutical provided support in the form of salary for author KC, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘author contributions’ section.
References
- 1.Brenowitz WD, Keene CD, Hawes SE, Hubbard RA, Longstreth WT Jr., Woltjer RL, et al. Alzheimer's disease neuropathologic change, Lewy body disease, and vascular brain injury in clinic- and community-based samples. Neurobiol Aging 2017. May;53:83–92. 10.1016/j.neurobiolaging.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson RS, Capuano AW, Bennett DA, Schneider JA, Boyle PA. Temporal course of neurodegenerative effects on cognition in old age. Neuropsychology 2016. July;30(5):591–9. 10.1037/neu0000282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caselli RJ, Beach TG, Knopman DS, Graff-Radford NR. Alzheimer Disease: Scientific Breakthroughs and Translational Challenges. Mayo Clin Proc 2017. June;92(6):978–94. 10.1016/j.mayocp.2017.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White LR, Edland SD, Hemmy LS, Montine KS, Zarow C, Sonnen JA, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology 2016. March 15;86(11):1000–8. 10.1212/WNL.0000000000002480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology 2015. August 11;85(6):535–42. 10.1212/WNL.0000000000001831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology 2015. January 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007. December 11;69(24):2197–204. 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 8.Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer's disease. Acta Neuropathol Commun 2018. May 1;6(1):33 10.1186/s40478-018-0531-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol 2010. January;20(1):66–79. 10.1111/j.1750-3639.2008.00244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Josephs KA, Dickson DW, Tosakulwong N, Weigand SD, Murray ME, Petrucelli L, et al. Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer's disease: a longitudinal retrospective study. Lancet Neurol 2017. November;16(11):917–24. 10.1016/S1474-4422(17)30284-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuang DW, Riekse RG, Purganan KM, David AC, Montine TJ, Schellenberg GD, et al. Lewy body pathology in late-onset familial Alzheimer's disease: a clinicopathological case series. J Alzheimers Dis 2006. August;9(3):235–42. [DOI] [PubMed] [Google Scholar]
- 12.Uchikado H, !Lost Data, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 2006. July;65(7):685–97. 10.1097/01.jnen.0000225908.90052.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton RL. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 2000. July;10(3):378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand E, Lechowicz W, Szpak GM, Lewandowska E, Dymecki J, Wierzba-Bobrowicz T. Limbic neuropathology in idiopathic Parkinson's disease with concomitant dementia. Folia Neuropathol 2004;42(3):141–50. [PubMed] [Google Scholar]
- 15.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, et al. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson's disease. Acta Neuropathol (Berl) 2003. July;106(1):83–8. [DOI] [PubMed] [Google Scholar]
- 16.Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson's disease. Acta Neuropathol (Berl) 2000. September;100(3):285–90. [DOI] [PubMed] [Google Scholar]
- 17.Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology 2000. May 23;54(10):1916–21. 10.1212/wnl.54.10.1916 [DOI] [PubMed] [Google Scholar]
- 18.Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson's disease. Acta Neuropathol (Berl) 2000. September;100(3):285–90. [DOI] [PubMed] [Google Scholar]
- 19.Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 2005. April 26;64(8):1404–10. 10.1212/01.WNL.0000158422.41380.82 [DOI] [PubMed] [Google Scholar]
- 20.Jellinger KA. Significance of brain lesions in Parkinson disease dementia and Lewy body dementia. Front Neurol Neurosci 2009;24:114–25. 10.1159/000197890 [DOI] [PubMed] [Google Scholar]
- 21.Burack MA, Hartlein J, Flores HP, Taylor-Reinwald L, Perlmutter JS, Cairns NJ. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology 2010. January 5;74(1):77–84. 10.1212/WNL.0b013e3181c7da8e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van DV, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 2012. October;72(4):587–98. 10.1002/ana.23659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabbagh MN, Adler CH, Lahti TJ, Connor DJ, Vedders L, Peterson LK, et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord 2009. July;23(3):295–7. 10.1097/WAD.0b013e31819c5ef4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Compta Y, Parkkinen L, O'Sullivan SS, Vandrovcova J, Holton JL, Collins C, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain 2011. May;134(Pt 5):1493–505. 10.1093/brain/awr031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008. April 30;23(6):837–44. 10.1002/mds.21956 [DOI] [PubMed] [Google Scholar]
- 26.Akhtar RS, Xie SX, Brennan L, Pontecorvo MJ, Hurtig HI, Trojanowski JQ, et al. Amyloid-Beta Positron Emission Tomography Imaging of Alzheimer's Pathology in Parkinson's Disease Dementia. Mov Disord Clin Pract 2016. July;3(4):367–75. 10.1002/mdc3.12290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deramecourt V, Bombois S, Maurage CA, Ghestem A, Drobecq H, Vanmechelen E, et al. Biochemical staging of synucleinopathy and amyloid deposition in dementia with Lewy bodies. J Neuropathol Exp Neurol 2006. March;65(3):278–88. 10.1097/01.jnen.0000205145.54457.ea [DOI] [PubMed] [Google Scholar]
- 28.Graff-Radford J, Aakre J, Savica R, Boeve B, Kremers WK, Ferman TJ, et al. Duration and Pathologic Correlates of Lewy Body Disease. JAMA Neurol 2017. March 1;74(3):310–5. 10.1001/jamaneurol.2016.4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord 2002. October;16(4):203–12. [DOI] [PubMed] [Google Scholar]
- 30.Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology 2015. August;35(4):354–89. 10.1111/neup.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeith I, Taylor JP, Thomas A, Donaghy P, Kane J. Revisiting DLB Diagnosis: A Consideration of Prodromal DLB and of the Diagnostic Overlap With Alzheimer Disease. J Geriatr Psychiatry Neurol 2016. September;29(5):249–53. 10.1177/0891988716656083 [DOI] [PubMed] [Google Scholar]
- 32.Lebouvier T, Delrieu J, Evain S, Pallardy A, Sauvaget A, Letournel F, et al. [Dementia: Where are the Lewy bodies?]. Rev Neurol (Paris) 2013. November;169(11):844–57. [DOI] [PubMed] [Google Scholar]
- 33.Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol 2010. March;257(3):359–66. 10.1007/s00415-009-5324-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, et al. Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology 1998. August;51(2):351–7. 10.1212/wnl.51.2.351 [DOI] [PubMed] [Google Scholar]
- 35.Kramberger MG, Auestad B, Garcia-Ptacek S, Abdelnour C, Olmo JG, Walker Z, et al. Long-Term Cognitive Decline in Dementia with Lewy Bodies in a Large Multicenter, International Cohort. J Alzheimers Dis 2017;57(3):787–95. 10.3233/JAD-161109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenowitz WD, Hubbard RA, Keene CD, Hawes SE, Longstreth WT Jr., Woltjer RL, et al. Mixed neuropathologies and estimated rates of clinical progression in a large autopsy sample. Alzheimers Dement 2017. June;13(6):654–62. 10.1016/j.jalz.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraybill ML, Larson EB, Tsuang DW, Teri L, McCormick WC, Bowen JD, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology 2005. June 28;64(12):2069–73. 10.1212/01.WNL.0000165987.89198.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferman TJ, Aoki N, Crook JE, Murray ME, Graff-Radford NR, van Gerpen JA, et al. The limbic and neocortical contribution of alpha-synuclein, tau, and amyloid beta to disease duration in dementia with Lewy bodies. Alzheimers Dement 2018. March;14(3):330–9. 10.1016/j.jalz.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 2009. June;117(6):613–34. 10.1007/s00401-009-0538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005. December 27;65(12):1863–72. 10.1212/01.wnl.0000187889.17253.b1 [DOI] [PubMed] [Google Scholar]
- 41.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017. July 4;89(1):88–100. 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savica R, Beach TG, Hentz JG, Sabbagh MN, Serrano GE, Sue LI, et al. Lewy Body pathology in Alzheimer's Disease: A clinicopathological prospective study. Acta Neurol Scand 2018. September 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology 2015. August;35(4):354–89. 10.1111/neup.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging 1997. July;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- 45.Shprecher DR, Adler CH, Zhang N, Hentz JG, Serrano GE, Dugger BN, et al. Predicting alpha-synuclein pathology by REM sleep behavior disorder diagnosis. Parkinsonism Relat Disord 2018. May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adler CH, Hentz JG, Shill HA, Sabbagh MN, Driver-Dunckley E, Evidente VG, et al. Probable RBD is increased in Parkinson's disease but not in essential tremor or restless legs syndrome. Parkinsonism Relat Disord 2011. July;17(6):456–8. 10.1016/j.parkreldis.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med 2013. August;14(8):754–62. 10.1016/j.sleep.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boeve BF, Molano JR, Ferman TJ, Lin SC, Bieniek K, Tippmann-Peikert M, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med 2013. May 15;9(5):475–80. 10.5664/jcsm.2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rongve A, Soennesyn H, Skogseth R, Oesterhus R, Hortobagyi T, Ballard C, et al. Cognitive decline in dementia with Lewy bodies: a 5-year prospective cohort study. BMJ Open 2016. February 29;6(2):e010357 10.1136/bmjopen-2015-010357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker Z, McKeith I, Rodda J, Qassem T, Tatsch K, Booij J, et al. Comparison of cognitive decline between dementia with Lewy bodies and Alzheimer's disease: a cohort study. BMJ Open 2012;2:e000380 10.1136/bmjopen-2011-000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams MM, Xiong C, Morris JC, Galvin JE. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology 2006. December 12;67(11):1935–41. 10.1212/01.wnl.0000247041.63081.98 [DOI] [PubMed] [Google Scholar]
- 52.Hanyu H, Sato T, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Differences in clinical course between dementia with Lewy bodies and Alzheimer's disease. Eur J Neurol 2009. February;16(2):212–7. 10.1111/j.1468-1331.2008.02388.x [DOI] [PubMed] [Google Scholar]
- 53.Costa A, Carlesimo GA, Caltagirone C, Mazzone P, Pierantozzi M, Stefani A, Peppe A. Effects of deep brain stimulation of the peduncolopontine area on working memory tasks in patients with Parkinson's disease. Parkinsonism Relat Disord. 2010. January;16(1):64–7. 10.1016/j.parkreldis.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 54.Spires-Jones TL, Attems J, Thal DR. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol 2017. August;134(2):187–205. 10.1007/s00401-017-1709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci 2010. May 26;30(21):7281–9. 10.1523/JNEUROSCI.0490-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winslow AR, Moussaud S, Zhu L, Post KL, Dickson DW, Berezovska O, et al. Convergence of pathology in dementia with Lewy bodies and Alzheimer's disease: a role for the novel interaction of alpha-synuclein and presenilin 1 in disease. Brain 2014. July;137(Pt 7):1958–70. 10.1093/brain/awu119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaneko H, Kakita A, Kasuga K, Nozaki H, Ishikawa A, Miyashita A, et al. Enhanced accumulation of phosphorylated alpha-synuclein and elevated beta-amyloid 42/40 ratio caused by expression of the presenilin-1 deltaT440 mutant associated with familial Lewy body disease and variant Alzheimer's disease. J Neurosci 2007. November 28;27(48):13092–7. 10.1523/JNEUROSCI.4244-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishikawa A, Piao YS, Miyashita A, Kuwano R, Onodera O, Ohtake H, et al. A mutant PSEN1 causes dementia with Lewy bodies and variant Alzheimer's disease. Ann Neurol 2005. March;57(3):429–34. 10.1002/ana.20393 [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg CK, Pericak-Vance MA, Saunders AM, Gilbert JR, Gaskell PC, Hulette CM. Lewy body and Alzheimer pathology in a family with the amyloid-beta precursor protein APP717 gene mutation. Acta Neuropathol 2000. August;100(2):145–52. [DOI] [PubMed] [Google Scholar]
- 60.Lantos PL, Ovenstone IM, Johnson J, Clelland CA, Roques P, Rossor MN. Lewy bodies in the brain of two members of a family with the 717 (Val to Ile) mutation of the amyloid precursor protein gene. Neurosci Lett 1994. May 19;172(1–2):77–9. [DOI] [PubMed] [Google Scholar]
- 61.Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, Schmidt ML, et al. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer's disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 1998. November;153(5):1365–70. 10.1016/s0002-9440(10)65722-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leverenz JB, Fishel MA, Peskind ER, Montine TJ, Nochlin D, Steinbart E, et al. Lewy body pathology in familial Alzheimer disease: evidence for disease- and mutation-specific pathologic phenotype. Arch Neurol 2006. March;63(3):370–6. 10.1001/archneur.63.3.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bugiani O, Giaccone G, Piccardo P, Morbin M, Tagliavini F, Ghetti B. Neuropathology of Gerstmann-Straussler-Scheinker disease. Microsc Res Tech 2000. July 1;50(1):10–5. [DOI] [PubMed] [Google Scholar]
- 64.Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain 2010. February;133(Pt 2):540–56. 10.1093/brain/awp280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med 2013. March 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murray ME, Ferman TJ, Boeve BF, Przybelski SA, Lesnick TG, Liesinger AM, et al. MRI and pathology of REM sleep behavior disorder in dementia with Lewy bodies. Neurology 2013. November 5;81(19):1681–9. 10.1212/01.wnl.0000435299.57153.f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chem Senses 2011. May;36(4):367–73. 10.1093/chemse/bjq139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujishiro H, Iseki E, Nakamura S, Kasanuki K, Chiba Y, Ota K, et al. Dementia with Lewy bodies: early diagnostic challenges. Psychogeriatrics 2013. June;13(2):128–38. 10.1111/psyg.12005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly because of identifying information contained in the dataset. Data are available from Banner Sun Health Research Institute (www.brainandbodydonationprogram.org) for researchers who meet the criteria for access to confidential data.