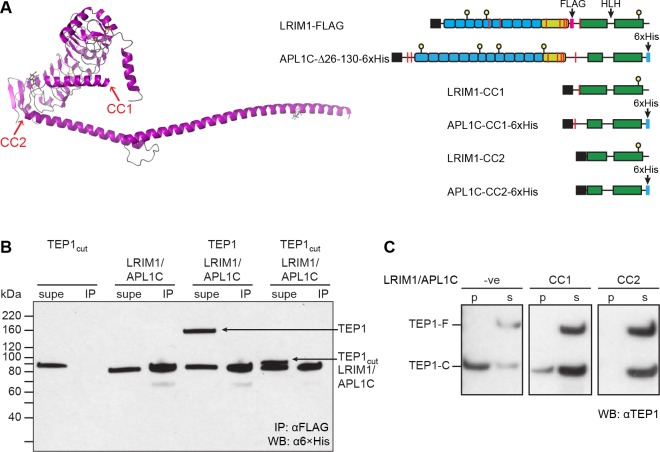
Fig 3. Disulfide-stabilized TEP1cut does not bind LRIM1/APL1C.
(A) Model of the domain structure of LRIM1/APL1C (LRIM1 only, magenta) indicating the location of C-terminal truncations CC1 and CC2. A schematic diagram of LRIM1-FLAG/APL1C-Δ26-130-6×His construct and coiled-coil domain constructs CC1 and CC2, coiled-coil shown in green, cysteines as red bars, N-linked glycosylation site as yellow ball, 6×His tag in blue. (B) α6×His Western blot of supernatant (supe) and αFLAG immunoprecipitate (IP) of TEP1*S1-K969C/Y1275C seven days post-cleavage. The first sample is wt TEP1*S1 immediately following cleavage. Both uncleaved and cleaved TEP1 remain in the supernatatant and do not co-immunoprecipitate with LRIM1/APL1C. (C) αTEP1 Western blot of precipitate (p) and soluble (s) fractions for TEP1*S1 full-length (TEP1-F) and cleaved form (TEP1-C) after 24 h incubation with LRIM1/APL1C-CC1 and LRIM1/APL1C-CC2.