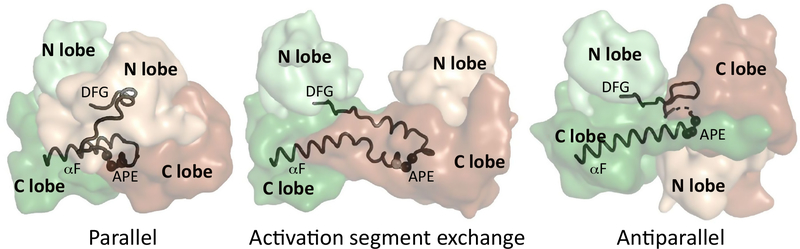
Figure 3. Different Types of Kinase Domain Dimers.
In a parallel hetero dimeric kinase domain complex, the dimerization interface is mainly formed by the G helices of both kinases and the conformation of the APE motif stays as in the kinase monomers. In contrast, in the activation segment exchange model, the activation segments between the upstream and the downstream kinases are swapped; therefore, the APE motif undergoes a major conformational change. In the antiparallel binding mode, the APE motif region also changes its position in order to interact with the ATP-bound G loop of the upstream kinase. This rearrangement is required to expose the rest of the activation loop of the downstream kinase to the active site of the upstream kinase. The activator kinase is colored in brown, the receiver kinase green, and the N and C lobes are shown in light ordarker colors, respectively. The different types of kinase dimers are shown as examples using the following structures from the PDB: parallel-KSR2-MEK1, PDB ID: 2Y4I; activation segment exchange-CHK2, PDB ID: 2CN5; antiparallel-ERK2-RSK1(CTK), PDB ID: 4NIF.