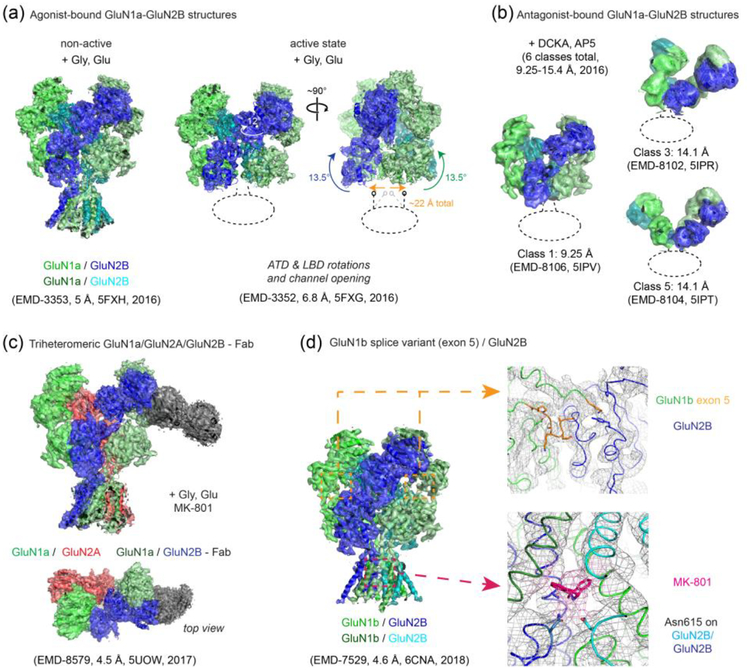
Figure 2. Single particle cryo-EM studies on the rat and Xenopus NMDARs.
(a) Multiple conformational states of the rat GluN1-GluN2B NMDARs bound to the agonists, glycine and glutamate. The cryo-EM density maps and the molecular models for the GluN1 subunits are colored green and light green whereas those for the GluN2B subunits are colored cyan and dark blue. Transitioning from the non-active state to the active state requires ‘opening’ of the bi-lobed structure of the GluN2B ATD and inter-subunit reorientation of the GluN1 and GluN2B ATDs by ~12°, which translates to rotation movement of the GluN1-GluN2B LBD heterodimers by ~13° and onto the gate of the TMD channel (arrows show movement from “non-active 2” state to “active” state [[61]]). Unresolved TMDs are marked with dotted ellipses/lines. (b) Binding of the antagonists, DCKA and AP5, leads to large movements in the extracellular domains as shown by selected 3D classes of the Xenopus GluN1a-GluN2B NMDARs with different conformations and the disrupted pattern of the “dimer of dimers” assembly. The color code is as in panel (a). (c) The overall assembly pattern of the tri-heteromeric Xenopus GluN1a-GluN2A-GluN2B NMDARs is similar to that of the di-heteromeric GluN1-GluN2B NMDARs in that the GluN1-GluN2-GluN1-GluN2 subunit arrangement is conserved. Binding of anti-GluN2B Fab (dark gray) facilitated single particle analysis by distinguishing the GluN2B subunit (dark blue) from the GluN2A subunit (red). (d) The cryo-EM structure of the GluN1b-GluN2B NMDAR splicing variant containing exon 5 in the GluN1b subunit shows that the exon 5-encoded motif (orange) is located at the ATDLBD interface and interacts with both the GluN1b LBD and GluN2B LBD to strengthen the hetero-dimeric interaction. This structure also showed cryo-EM density for a channel blocker, MK-801, and interacting residues such as GluN2B Asn615 (shown as sticks) in the TMD channel. Similar density for MK-801 was also observed in the cryo-EM structure of the tri-heteromeric Xenopus GluN1a-GluN2A-GluN2B NMDARs. Resolutions of the EM maps (in Å) for any given class (with EMD code) are stated as in the EM Data Bank, which were determined by the current gold-standard Fourier Shell Correlation (FSC) cutoff at 0.143.