Abstract
As β3‐adrenoceptors were first demonstrated to be expressed in adipose tissue they have received much attention for their metabolic effects in obesity and diabetes. After the existence of this subtype had been suggested to be present in the heart, studies focused on its role in cardiac function. While the presence and functional role of β3‐adrenoceptors in the heart has not uniformly been detected, there is a broad consensus that they become up‐regulated in pathological conditions associated with increased sympathetic activity such as heart failure and diabetes. When detected, the β3‐adrenceptor has been demonstrated to mediate negative inotropic effects in an inhibitory G protein‐dependent manner through the NO–cGMP–PKG signalling pathway. Whether these negative inotropic effects provide protection from the adverse effects induced by overstimulation of β1/β2‐adrenoceptors or in themselves are potentially harmful is controversial, but ongoing clinical studies in patients with congestive heart failure are testing the hypothesis that β3‐adrenceptor agonism has a beneficial effect.
Linked Articles
This article is part of a themed section on Adrenoceptors—New Roles for Old Players. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.14/issuetoc
Abbreviations
- Gi
inhibitory G protein
- PTX
pertussis toxin
1. INTRODUCTION
β‐Adrenoceptors are essential regulators of cardiac function (Brodde & Michel, 1999). Most studies in the heart involved β1‐ and β2‐adrenoceptors, and the existence of a third subtype became established unequivocally with the cloning of the human gene encoding the β3‐adrenoceptor (Emorine et al., 1989). While originally proposed as a regulator of thermogenesis and lipolysis in rodents (Harms, Zaagsma, & van der Wal, 1974), β3‐adrenoceptors in the heart have been widely studied (Gauthier, Langin, & Balligand, 2000; Michel, Harding, & Bond, 2011), particularly with regard to them as a potential target for the treatment of congestive heart failure (Rasmussen, Figtree, Krum, & Bundgaard, 2009). As the role of β3‐adrenoceptors in the human heart has been questioned (Mo, Michel, Lee, Kaumann, & Molenaar, 2017), this manuscript discusses the presence and potential physiological and pathological role of β3‐adrenoceptors in the heart with a special focus on the human heart.
2. EXPRESSION AND FUNCTION OF β3‐ADRENOCEPTORS IN NORMAL HEART
The heart is composed of various cell types, including cardiomyocytes, fibroblasts and smooth muscle cells, and endothelial cells of the coronary arteries and intramural blood vessels. This means that studies not isolating specific cell types may have underappreciated findings limited to some of these cell types, as they get diluted in overall cardiac preparations. For instance, β3‐adrenoceptors expressed in specific cell types such as vascular endothelial cells may be relevant for cardiac function but barely detectable in homogenates of cardiac biopsies.
A second major limitation of much of the data available relates to the limited specificity of the tools being used. For instance, many of the antibodies do not exhibit the promised target specificity when tested under stringent conditions (see Section 2.2). Similar problems exist for many of the pharmacological tools. For instance, the frequently used agonist BRL 37,344 has only poor selectivity for β3‐ over β2‐adrenoceptors (Cernecka, Sand, & Michel, 2014), and the partial β3‐adrenoceptor agonist CGP 12,177 is an antagonist at the orthotopic site of the β1‐adrenoceptor and an agonist at a heterotopic site (see Section 2.4). Therefore, many investigators have chosen to use differential antagonism by nadolol or propranolol (assumed to block β1‐ and β2‐adrenoceptors) rather than SR 59,230 (assumed to block all three subtypes). While SR 59,230 is often referred to as β3‐adrenoceptor‐selective, several studies have shown that it has comparable affinity for all three subtypes (Baker, 2010; Hoffmann, Leitz, Oberdorf‐Maass, Lohse, & Klotz, 2004; Niclauß, Michel‐Reher, Alewijnse, & Michel, 2006); moreover, it can be a partial agonist for β3‐adrenoceptor‐mediated cAMP formation and a stronger biased agonist for other signalling pathways of this receptor (Hutchinson, Sato, Evans, Christopoulos, & Summers, 2005). Additionally, agonists may already activate receptors at concentrations considerably lower than those needed to occupy 50% of them, a phenomenon called receptor reserve, which is particularly prominent in the heart (Brown et al., 1992). Therefore, the possibility that high concentrations/doses of moderately selective ligands may have off‐target effects needs to be considered, and very few if any of the in vivo studies have determined exposure at the dose levels being used. All of these issues can become exacerbated when single concentrations/doses are used. Thus, many of the experiments discussed here do not directly allow conclusions to be drawn on the involvement of β3‐adrenoceptors and only do so indirectly when inhibition profiles of antagonists with high affinity for the β3‐adrenoceptor were compared. These selectivity issues limit the interpretation of the majority of the data available, not only those related to cardiac function but also in the overall β3‐adrenoceptor field. These limitations should be kept in mind in the interpretation of all data discussed hereafter.
The expression and function of β3‐adrenoceptors appears to differ depending on the species studied (Gauthier et al., 1999). The β3‐adrenoceptor genes of rats and mice, the most frequently used species in non‐clinical β3‐adrenoceptor‐related studies, share 79% homology with the human gene (Rozec & Gauthier, 2006), including splice variants of the mouse but not human gene (Evans, Papaioannou, Hamilton, & Summers, 1999). Furthermore, the potency/affinity of many ligands also differ between species (Cernecka et al., 2014). For example, the potency of the agonist CL 316,243 (Strosberg, 1997) and of the antagonist L 748,337 (van Wieringen, Michel‐Reher, Hatanaka, Ueshima, & Michel, 2013) differ more than 10‐fold between rodent and human β3‐adrenoceptors. These interspecies differences complicate the extrapolation of animal data to human physiology.
2.1. mRNA
The structure of the β3‐adrenoceptor gene differs between species with the mouse orthologue giving rise to two splice variants, β3a and β3b, that differ in their 3′ untranslated regions (Evans et al., 1999). The cardiac expression of β3‐adrenoceptor mRNA has repeatedly been studied in healthy rats, mice, dogs, and humans. Interpretation of the reported data is hampered by several limitations. Most importantly, most studies have been based on cardiac extracts. This gives potential for both false positive and false negative findings. False negative findings may occur if the target gene is expressed only in a small subset of cells that yields an insufficiently strong signal to allow robust detection in the overall extract. False positive results may result from the presence of cell types other than cardiomyocytes such as fibroblasts, cells of intra‐cardiac blood vessels and adipose tissue. For example, one of the studies demonstrating the presence of β3‐adrenoceptors in human heart also reported a strong signal for UCP‐1 (Krief et al., 1993); as UCP‐1 is a protein typically found in brown adipose tissue, this may indicate contamination with adipose tissue. The potential for false positives becomes more likely if a very sensitive technique is applied (e.g., 30 PCR cycles; Gauthier, Tavernier, Charpentier, Langin, & Le Marec, 1996). The use of trabecular strips in place of whole heart extracts may reduce this issue but not fully exclude it. More informative are techniques focusing solely on cardiomyocytes, for example, those based on laser‐caption dissection microscopy (Moniotte, Vaerman, et al., 2001). Moreover, human samples from normal hearts typically did not come from fully healthy people but rather from those without overt heart failure. As various diseases are associated with an up‐regulation of cardiac β3‐adrenoceptors (see Section 3), it is possible that the values reported do not reflect the situation in truly healthy people.
Some studies in rats (Evans, Papaioannou, Anastasopoulos, & Summers, 1998; Gauthier et al., 1999; Muzzin et al., 1991; Rautureau et al., 2002) but not others (Dinçer et al., 2001; Ozakca et al., 2013; Zhao et al., 2013) failed to detect cardiac β3‐adrenoceptor mRNA; interestingly, the former two studies (from the same group) reported an increase in β3‐adrenoceptor expression upon a chronic infusion of isoprenaline (Ozakca et al., 2013) or in experimental diabetes (Dinçer et al., 2001). Studies in mouse heart have reported a lack of β3‐adrenoceptor mRNA (Nahmias et al., 1991) or a limited expression that was mostly attributable to the β3a splice variant (Evans et al., 1999); one genome‐wide study reported the presence of β3‐adrenoceptor mRNA in hearts of male but not female mice (Li et al., 2017). In contrast, β3‐adrenoceptor mRNA was found to be present in dog heart (Gauthier et al., 1999). Findings in human heart are more complex; two studies that did not specify which part of the heart had been tested reported an absence of β3‐adrenoceptor mRNA (Mak et al., 1996; Uhlen et al., 2015). One of these groups, in a concomitant publication, were also unable to detect β3‐adrenoceptor mRNA in samples from left ventricle and left atrium of two patients (Lindskog et al., 2015); the ventricular samples came from subjects with cardiac hypertrophy or postpartum cardiomyopathy and the atrial samples from those with atrial fibrillation, but there is no explicit information as to whether these samples were also used in the other study from this lab (Uhlen et al., 2015). In a different study, β3‐adrenoceptor mRNA was found to be present at low levels in the atrium and ventricle (Krief et al., 1993), and these low levels in the atrium were confirmed by Berkowitz et al. (1995), but they did not detect β3‐adrenoceptor mRNA in the ventricle. Again β3‐adrenoceptor mRNA has been detected in endomyocardial biopsies from normal individuals (Moniotte, Vaerman, et al., 2001) or transplant patients (Gauthier et al., 1996). While the expression in transplant patients was similar to that in healthy controls, it was much lower than that of β1‐ or β2‐adrenoceptors (Moniotte, Vaerman, et al., 2001). Moreover, there is a possibility that some of the older studies may have been false negative results because of the use of techniques that are less sensitive than qPCR. Thus, the present data only allow us to conclude cardiac β3‐adrenoceptor mRNA expression is too low to be consistently detected.
2.2. Protein
The detection of β3‐adrenoceptors at the protein level is hampered by the limited validity of the tools available. Standard radioligands only have a low affinity for β3‐adrenoceptors (Niclauß et al., 2006), and the only selective radioligand, [3H]‐L 748,337, appears useful for human but not rodent β3‐adrenoceptor labelling due to species differences in affinity (van Wieringen et al., 2013). Moreover, most antibodies available exhibit little target specificity (Pradidarcheep et al., 2009) and those that are specific are only valid for selected species and/or applications (Cernecka, Ochodnicky, Lamers, & Michel, 2012; Cernecka, Pradidarcheep, Lamers, Schmidt, & Michel, 2014; Guillaume et al., 1994). Therefore, only limited data with technical validity exist on cardiac β3‐adrenoceptor expression at the protein level.
While only partly based on validated antibodies, β3‐adrenoceptor protein expression has been detected in mouse and sheep heart (Bundgaard et al., 2010), rat heart (Amour et al., 2008; Dinçer et al., 2001; Haley, Thackeray, Kolajova, Thorn, & DaSilva, 2015; Jiang et al., 2015), human atrium (Chamberlain et al., 1999), and human ventricle from patients with cardiomyopathy (De Matteis et al., 2002). One study, using an antibody validated as selective for β3‐ compared to β1‐ and β2‐adrenoceptors, demonstrated a marked up‐regulation of β3‐adrenoceptor protein in human heart failure and a considerable down‐regulation in the denervated heart (Moniotte, Vaerman, et al., 2001), whereas another reported an up‐regulation in a streptozotocin‐based rat model of type 1 diabetes (Amour et al., 2008).
2.3. Signalling responses
Despite the universal coupling of β3‐adrenoceptors to the stimulation of cAMP formation (Bylund et al., 1994), surprisingly, little information is available on β3‐adrenoceptor‐stimulated cAMP formation in the heart. CGP 12,177 was reported to stimulate AC activity in rat cardiac membranes in a propranolol‐resistant manner (Scarpace, Matheny, & Thümer, 1999). Later work from others demonstrated that this cAMP response to CGP 12,177 in rat and human heart is not mediated by the β3‐adrenoceptor but rather by an allosteric site of the β1‐adrenoceptor (Kaumann & Molenaar, 1997). In canine heart, neither BRL 37,344 nor CGP 12,177 stimulated AC activity (Tavernier, Galitzky, Bousquet‐Melou, Montastruc, & Berlan, 1992). In human heart, stimulation of cAMP formation by isoprenaline was fully blocked by propranolol (Ikezono, Michel, Zerkowski, Beckeringh, & Brodde, 1987). Taken together, these data demonstrate that β3‐adrenoceptors are to a large extent not involved the β‐adrenoceptor‐mediated formation of cAMP in the heart in rats, dogs, or humans.
β3‐adrenoceptors can also couple to the inhibitory G protein (Gi) to inhibit AC (Germack & Dickenson, 2006). Based on the effects of pertussis toxin (PTX) on negative inotropic responses, Gi‐coupling of β3‐adrenoceptors are thought to couple to Gi in mouse heart in response to the agonists isoprenaline and CL 316,243 in β1/β2 double knockout mice (Devic, Xiang, Gould, & Kobilka, 2001), BRL 37,344 in dog (Cheng et al., 2001), and human hearts (Gauthier et al., 1996). PTX also blocked the inhibition of Ca2+ channels induced by BRL 37,344 in the rat heart (Zhang et al., 2005). While we are not aware of any studies demonstrating an inhibitory effect evoked by β3‐adrenoceptor stimulation on cAMP formation in the heart, several investigators have demonstrated inhibition of cardiac responses to β3‐adrenoceptor agonists by PTX, thereby indirectly demonstrating coupling to Gi. For example, this includes inhibition of L‐type Ca2+ channels in rat (Zhang et al., 2005) and dog models of heart failure (Cheng et al., 2001) and negative inotropic effects in endomyocardial biopsies from transplant patients (Gauthier et al., 1996).
While studies in rat (Zhang et al., 2005) and dog models of heart failure (Cheng et al., 2001) have demonstrated inhibition of Ca2+ channels by β3‐adrenoceptor stimulation, experiments in human atrium have suggested stimulation of these channels (Skeberdis et al., 2008). However, it is possible that this latter effect is an artefact, observable only at unphysiologically low temperatures (Christ, Molenaar, Klenowski, Ravens, & Kaumann, 2011). Cardiac β3‐adrenoceptors have also been reported to inhibit the slow delayed rectifier K+ current in guinea pig cardiomyocytes (Bosch et al., 2002).
A detailed study on endomyocardial biopsies from human cardiac transplant patients has demonstrated that the negative inotropic effects of BRL 37,344 were abolished in the presence of NOS inhibitors (Gauthier et al., 1998). Moreover, they were associated with the formation of NO and intracellular cGMP. Immunohistochemical experiments demonstrated the presence of endothelial NOS (eNOS) both in cardiomyocytes and in endothelial cells. NO formation in response to β3‐adrenoceptor stimulation of human atria and ventricles has also been reported by others (Brixius et al., 2004; Napp et al., 2009). This was attributed to eNOS translocation in the right atrium, but to eNOS phosphorylation at serine 1177 and dephosphorylation at serine 114 in the left ventricle. While most studies have focused on eNOS, nNOS has been also implicated in cardiac effects of β3‐adrenoceptor stimulation (Amour et al., 2008; Watts et al., 2013). Finally, β3‐adrenoceptor stimulation has been reported to activate the Na+‐K+‐pump and increase the Na+‐K+‐pump current in rabbit ventricular myocytes, an effect abolished by the NOS inhibitor NG‐nitroarginine methyl ester (l‐NAME; Bundgaard et al., 2010). Both eNOS and the Na+‐K+‐pump can be inhibited in experimental diabetes, but this was restored by the β3‐adrenoceptor agonist CL 316,243 (Galougahi et al., 2015; Galougahi et al., 2016).
2.4. Inotropic and lusitropic responses
Data on potential inotropic and chronotropic effects of cardiac β3‐adrenoceptor stimulation are controversial, and it remains unclear whether these differences are due to variations within species, parts of the heart (atrium vs. ventricle), experimental methods used, and/or between healthy and diseased tissue. In this regard, two compounds have caused a lot of confusion. First, BRL 37,344 has often been used as a β3‐adrenoceptor agonist but has only limited selectivity over the β2‐adrenoceptor (Baker, 2010); accordingly, the effects of BRL 37,344 can occur concomitantly via β2‐ and β3‐adrenoceptors and can only be attributed to individual subtypes when appropriate antagonists are used (Mori, Miwa, Sakamoto, Nakahara, & Ishii, 2010). Second, CGP 12,177 is an antagonist at the orthosteric site of the β1‐ and β2‐adrenoceptor, an agonist at an allosteric site of the β1‐adrenoceptor, and a weak partial agonist at the orthosteric site of the β3‐adrenoceptor (Kaumann & Molenaar, 1997), which makes interpretation of its effects very difficult even when the orthosteric sites of β1‐ and β2‐adrenoceptors are blocked by antagonists.
Studies in rats have largely been based on in vitro experiments, either in isolated cardiac strips or Landendorff preparations, but in a few cases, effects on inotropy have been studied in vivo. However, the reported differences in effects remain largely unexplained by these methodological differences. In some cases, BRL 37,344 caused negative inotropic effects that were blocked by SR 59,230 but not by nadolol (Barbier, Mouas, Rannou‐Bekono, & Carré, 2007; Kayki‐Mutlu, Arioglu Inan, Ozakca, Ozcelikay, & Altan, 2014; Ozakca et al., 2013; Figure 1). However, others found that the negative inotropic effect of BRL 37,344 was only minor and not mimicked by CL 316,243 or SR 58,611 (Gauthier et al., 1999), suggesting that it was not related to β3‐adrenoceptor stimulation. BRL 37,344 was even reported to have a positive inotropic effect in one study, but this was blocked by propranolol and not mimicked by CL 316,243 (Shen, Cervoni, Claus, & Vatner, 1996), suggesting a β1‐ and/or β2‐adrenoceptor‐mediated effect. A lack of effect of BRL 37,344 was also reported in young rats, which turned into a negative inotropic effect in senescent animals, the latter in line with an increase in β3‐adrenoceptor protein expression (Birenbaum et al., 2008). Similarly, positive inotropic responses to isoprenaline were not affected by the antagonist cyanopindolol in healthy rats, but cyanopindolol at least partly restored decreased inotropic effects in diabetic animals, indirectly suggesting an involvement of β3‐adrenoceptors in the diabetic group (Amour et al., 2007). In contrast, cyanopindolol did not affect the lusitropic effect of isoprenaline (Amour et al., 2008). While the β1‐antagonist and alleged β3‐agonist nebivolol attenuated isoprenaline‐induced cardiac hypertrophy, the β1‐antagonist metoprolol did not (Ozakca et al., 2013). In contrast, BRL 37,344 was reported to increase right and left atrial mass index in a heart failure model, but the adrenoceptor subtype involved was not determined (Zhao et al., 2013).
Figure 1.
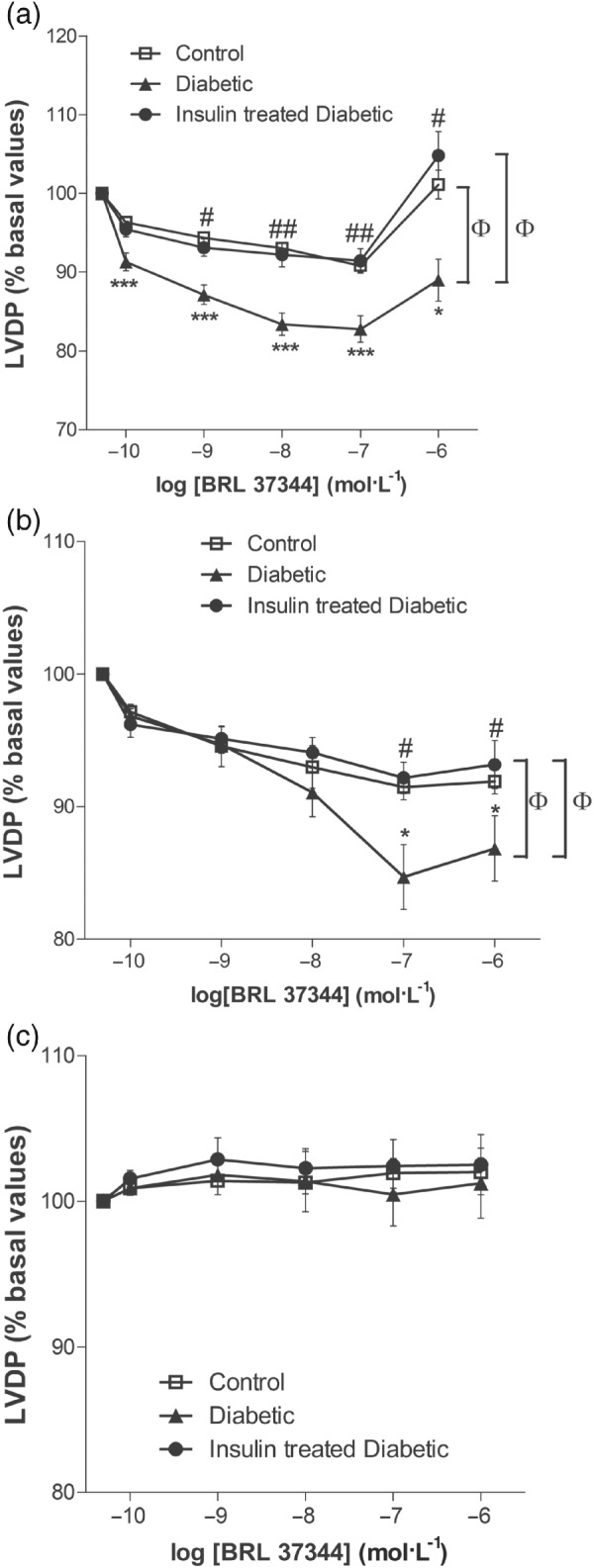
Effect of diabetes on the BRL 37344‐mediated negative inotropic effect in 8‐week diabetic rat heart. Shown are concentration–response curves for BRL 37344 in the absence of antagonists (a), presence of 10 μM nadolol (b), and presence of 0.1 μM SR 59,230 (c). *P < 0.05, ***P < 0.001, control versus diabetic; # P < 0.05, ## P < 0.01, diabetic versus insulin‐treated diabetic; ɸ P < 0.05, comparison of the different concentration–response curves by two‐way ANOVA. Adapted with permission from (Kayki‐Mutlu et al., 2014)
No evidence for any role for the β3‐adrenoceptor in the control of inotropy was found in normal mice (Heubach, Rau, Eschenhagen, Ravens, & Kaumann, 2002; Tavernier et al., 2003). In contrast, transgenic overexpression of the human β3‐adrenoceptor in mice resulted in increased inotropic responses to isoprenaline (Kohout et al., 2001). Independently generated mice with cardiac expression of the human β3‐adrenoceptor at levels comparable to those in human heart (Hermida et al., 2018) largely confirmed these observations: the β3‐adrenoceptor agonists CL 316,243 and SR 58,611 had a negative inotropic effect at low concentrations, but these vanished at higher concentrations (Tavernier et al., 2003). While nadolol inhibited the blunting of the negative inotropic effect of high agonist concentrations, bupranolol abolished both of these β3‐adrenoceptor agonist‐mediated effects, suggesting that the negative effects were mediated by the β3‐adrenoceptor, but their blunting by other subtypes. In guinea pig heart, CL 316,243 had a minor negative inotropic effect that was not mimicked by either BRL 37,344 or SR 58,611 (Gauthier et al., 1999). Within the same study, neither of the three compounds had any effect on cardiac contractility in ferrets (Gauthier et al., 1999).
Findings reported in the canine heart are contradictory. While one study found positive inotropic effects of BRL 37,344 and CL316,243 (Shen et al., 1996), another found negative inotropic effects of both the agonists and of SR 58,611, but their order of potency differed considerably from that observed in human heart, suggesting that this was not a β3‐adrenoceptor response (Gauthier et al., 1999). A more recent study used a different approach, that is, did not test agonists but rather the antagonist L 748,337 (Morimoto, Hasegawa, Cheng, Little, & Cheng, 2004). This compound had minor positive inotropic effects in healthy dogs; in those with heart failure, the positive inotropic effects became larger; in contrast, nadolol had negative inotropic effects in dogs with heart failure, and in the presence of nadolol isoprenaline also had negative inotropic effects. These data were interpreted as a minor β3‐adrenoceptor‐mediated negative inotropic effects in healthy and an enhanced negative inotropic effect in heart failure (see Section 3.2). In monkeys (Macaca facicularis) or baboons (Papio anubis), CL 316,243 did not affect cardiac contractility under conditions where it had done so in dogs (Shen et al., 1996).
Studies in non‐failing human heart mostly reported lack of effect of β3‐adrenoceptor stimulation. An early study found that the positive inotropic effect of isoprenaline was fully blocked by propranolol (Ikezono et al., 1987), providing indirect evidence against a role for β3‐adrenoceptors. A lack of effect of β3‐adrenoceptor stimulation in human heart was also reported by others (Harding, 1997). Similarly, positive inotropic effects and increases in Ca2+ transients caused by BRL 37,344 were blocked by propranolol, suggesting mediation via β1‐ and/or β2‐adrenoceptors (Pott et al., 2003); as a positive control within the same study, activation of NOS was not blocked by propranolol. In a later study, the same group reported NO‐dependent, negative inotropic effects of BRL 37,344 in failing heart under conditions where β3‐adrenoceptor protein expression was increased (Napp et al., 2009).
Mirabegron, the most β3‐selective agonist in clinical use, had minor, cAMP‐mediated positive inotropic effects in human atrium, and these were converted into negative inotropic effects upon blockade of β1‐adrenoceptors (Figure 2; Mo et al., 2017). The negative inotropic effects of mirabegron in the presence of β1‐adrenoceptor inhibition were not sensitive to L 748,337, that is, occurred via an unclear molecular mechanism. The β1‐adrenoceptor‐mediated positive effect was related to indirect sympathomimetic activity by the authors, based on the phenylethanolamine structure of mirabegron. Earlier work by others had reported that positive inotropic effects of β3‐adrenoceptor agonists in human heart were limited to those with a phenylethanolamine structure (Sennitt et al., 1998).
Figure 2.
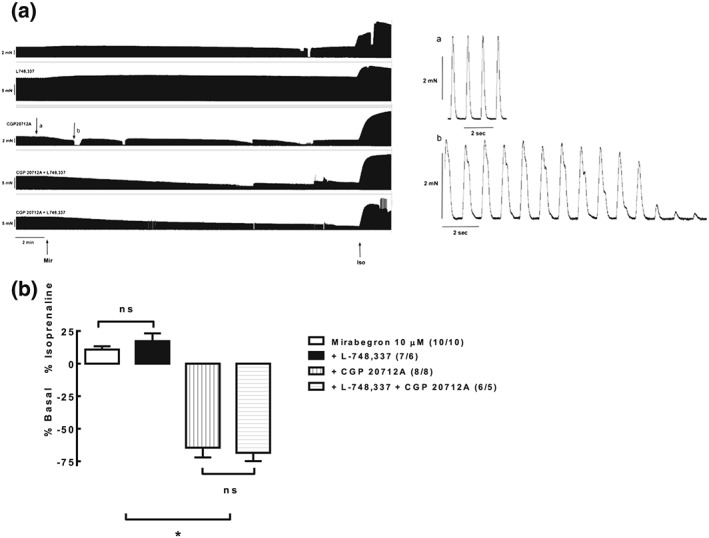
Effects of mirabegron on human atrial tone in the absence and presence of blockade of β1‐adrenoceptor antagonism (CGP 20,712), β3‐adrenoceptor antagonism (L 748,337), or combined agonism. ns: not statistically significant; *P < 0.05 as compared to data in the absence of CGP 20,712. Reproduced with permission from (Mo et al., 2017)
In contrast, another group has consistently reported negative inotropic effects of CL 316,243, BRL 37,344, SR 58,611 and nebivolol in endomycardial biopsies from non‐failing transplant recipients (Gauthier et al., 1996; Gauthier et al., 1999; Moniotte, Kobzik, et al., 2001; Rozec, Erfanian, Laurent, Trochu, & Gauthier, 2009); these were insensitive to nadolol but blocked by L 748,337. Despite an up‐regulation of β3‐adrenoceptor protein, these negative inotropic effects of BRL 37,344 were attenuated in failing hearts (Moniotte, Kobzik, et al., 2001), but a positive identification of β3‐adrenoceptors mediating these responses was not repeated within that study. Nevertheless, different investigators have also reported that BRL 37,344, SR 58,611, and CGP 12,177 have positive inotropic effects (Skeberdis et al., 2008); however, others have suggested that these are only observed at sub‐physiological temperatures (Christ et al., 2011). Finally, CGP 12,177 was reported to have positive inotropic effects in human atrium (Kaumann, 1996). However, several subsequent studies from these investigators showed that this effect was unrelated to the β3‐adrenoceptor and rather mediated by an allosteric site on the β1‐adrenoceptor (Kaumann & Molenaar, 1997). The same group has demonstrated positive inotropic effects of CGP 12,177 in murine heart that were insensitive to PTX and similarly found in β3‐adrenoceptor knockout mice (Kaumann et al., 1998; Oostendorp & Kaumann, 2000), substantiating the view that the positive inotropic effects of CGP 12,177 may primarily occur via an allosteric site on the β1‐adrenoceptor (Kaumann & Molenaar, 1997).
Taken together, these findings indicate that the role of β3‐adrenoceptors in the control of contractility in the non‐failing human heart is highly controversial with positive and negative inotropic effects as well as a lack of effect being reported. As the consistent detection of negative inotropic effects by one group was based on endomyocardial biopsies (i.e., ventricular tissue), whereas most studies not detecting effects were based on the atrium, it is possible that the role of β3‐adrenoceptors in the human heart differs between the atrium and ventricles.
2.5. Chronotropic responses
Early work in rats based on a variety of adrenergic agonists showed that agonists potently promoting adipocyte lipolysis, a β3‐adrenoceptor response, had low potency for enhancing rat atrial rate (Wilson, Wilson, Piercy, Sennitt, & Arch, 1984), suggesting that chronotropic responses in this species were not mediated by the β3‐adrenoceptor. Later work more directly confirmed this conclusion by demonstrating that positive chronotropic effects of BRL 37,344, CL 316, 243, and ZD 2079 were abolished by the combination of CGP 20,712 and ICI 118,551 (Malinowska & Schlicker, 1996). Moreover, all four stereoisomers of BRL 37,344 (Oriowo et al., 1996) as well as CL 316,243 (Malinowska & Schlicker, 1997) exhibited low potency for atrial rate stimulation. Several aryl propanolamines including L 739,574 also had low potency for affecting the atrial rate response as compared to that mediated by human β3‐adrenoceptor (Cohen, Bloomquist, Kriauciunas, Shuker, & Calligaro, 1999). In rats with cerebral infarction, CL 316,243 increased urinary bladder capacity but had only little effect on heart rate (Kaidoh et al., 2002). Others found little effect of CL 316,243 on heart rate in healthy rats at doses that prolonged the inter‐contraction intervals of the bladder (Takeda et al., 2002). Small chronotropic effects of BRL 37,344 and CL 316,243 were confirmed by additional investigators but markedly attenuated by SR 59,230 or L 748,337, an effect interpreted to occur secondary to β3‐adrenoceptor‐mediated vasodilation (Mori et al., 2010). The β1‐adrenoceptor antagonist and alleged β3‐adrenoceptor agonist nebivolol similarly decreased heart rate in an isoprenaline‐induced model of heart failure (Ozakca et al., 2013). Taken together, these results indicate that the β3‐adrenoceptor contributes only little to the control of heart rate in rats. In neonatal mouse cardiomyocytes from β1/β2 double knockout mice, isoprenaline and CL 316,243 produced negligible (about 5 beats·min−1) decreases in contraction rate, which were turned into larger (about 20 beats·min−1) increases upon treatment with PTX; CL 316,243 also reduced beating rate in wild‐type cardiomyocytes (Devic et al., 2001). In contrast, adrenaline and noradrenaline increased heart rate predominantly if not exclusively via β1‐adrenoceptors in atria from adult mice, whether or not animals had been pretreated with PTX (Heubach et al., 2002).
In vivo studies in dogs based on BRL 37,344 and CL 316,243 observed positive chronotropic effects, but these were abolished in animals with sino‐aortic denervation, indicating that the heart rate increases were a result of baroreflex activation (Tavernier et al., 1992). Others, using SR 58,611 as the β3‐adrenoceptor agonist, confirmed an increase in heart rate that disappeared after sino‐aortic denervation or propranolol or nadolol, thus also attributing it to peripheral vasodilation and baroreflex activation (Montastruc et al., 1999). An increased heart rate was also observed upon injection of CL 316, 243, performed as part of the safety monitoring in studies on ureteral tone (Murakami et al., 2000). Moreover, BRL 37,344‐induced increases in heart rate, in contrast to those induced by isoprenaline, persisted in the presence of propranolol; they were largely attributed to peripheral vasodilation, mostly in the vascular beds of the skin and adipose tissue (Shen, Zhang, & Vatner, 1994). However, this interpretation is problematic; while there may have been β3‐adrenoceptor‐mediated vasodilatation, any catecholamines being released upon baroreflex activation would be expected to ultimately act via cardiac β1‐adrenoceptors and thus should have been sensitive to propranolol. Similarly, part of the tachycardia induced by isoprenaline remained after administration of nadolol but disappeared after additional administration of SR 59,230 (Pelat et al., 2003). Taken together, positive chronotropic effects of β3‐adrenoceptor agonists can largely be attributed to peripheral vasodilation and resulting baroreflex activation; of note, all three β‐adrenoceptor subtypes can contribute to peripheral vasodilation (Guimaraes & Moura, 2001); their relative roles depend on species, vascular bed under investigation, and selectivity of tools used to study them.
Few investigators have directly explored the role of β3‐adrenoceptors in the regulation of human heart rate. In contrast to gene polymorphisms of the β1‐adrenoceptor, those of the β3‐adrenoceptor did not exhibit associations with resting heart rate in a cohort of more than 1,000 subjects of Chinese or Japanese descent (Ranade et al., 2002). Supratherapeutic doses of mirabegron (100–200 mg) increased heart rate in a Phase II dose‐ranging study (Chapple et al., 2013). In a follow‐up study in young, healthy volunteers treated with the 200 mg dose, this increase was confirmed, and was markedly attenuated by co‐administration of propranolol or bisoprolol (van Gelderen et al., 2017), suggesting that the β1‐adrenoceptor was largely involved in this response. Clinical studies with mirabegron, when used for the treatment of patients with overactive bladder syndrome, have detected only small if any increases in heart rate (Michel & Gravas, 2016; Rosa et al., 2018). In the light of the above findings in healthy volunteers (van Gelderen et al., 2017) and those in isolated human atrium (Mo et al., 2017), these responses to mirabegron can most likely be explained by peripheral vasodilation and/or indirect sympathomimetic effects. However, neither of these hypotheses has been proven in humans. Thus, there is only very limited evidence supporting a possible direct positive chronotropic of β3‐adrenoceptor in humans.
3. ALTERATIONS IN PATHOPHYSIOLOGY
3.1. Gender and ageing
Only one study has reported on possible sex differences; in a genome wide assessment of expression profiles, they found β3‐adrenoceptor mRNA in the heart of male but not female mice (Li et al., 2017). Findings in rat (Frazier, Schneider, & Michel, 2006; Kullmann et al., 2009) and human urinary bladder (Schneider, Fetscher, & Michel, 2011) suggest that the expression and function of β3‐adrenoceptors does not differ between genders.
The responsiveness of cardiac β1‐ and β2‐adrenoceptor declines with ageing, which has mainly been attributed to reductions in the expression of β1‐adrenoceptors and Gs protein in the ventricle of human heart, whereas an increase in Gi protein and decrease in the catalytic unit of AC appear to be involved (Brodde & Michel, 1999; Woo, Song, Xiao, & Zhu, 2015). Protein expression of β3‐adrenoceptors was up‐regulated in hearts from 24‐ as compared to 3‐months‐old rats (Birenbaum et al., 2008); this up‐regulation was associated with an attenuation of positive inotropic effects of dibutyryl‐cAMP by BRL 37,344 in the senescent but not the young adult animals. Moreover, NOS inhibition did not affect inotropic responses to isoprenaline in young animals, but the attenuated inotropic effect in senescent rats was at least partly restored by an NOS inhibitor. While expression levels of NOS isoforms were not determined in this study, increased expression of endothelial, neuronal, and inducible NOS in aged rat heart has been reported by others (Li et al., 2006; Piech, Dessy, Havaux, Feron, & Balligand, 2003). Therefore, it was suggested that blocking the β3‐adrenoceptor pathway may improve cardiac output in senescent heart, as stimulation of this subtype reduces β‐adrenoceptor‐mediated positive inotropy (Birenbaum et al., 2008). However, an alternative interpretation of the data could be that negative inotropic effects of β3‐adrenoceptor stimulation may protect the heart from adverse effects of excessive stimulation of β1/β2‐adrenoceptor activation; this different interpretation is the basis for the controversy of whether activation or inhibition of β3‐adrenoceptors would be a promising approach for the treatment of heart failure (see Section 4).
3.2. Heart failure
Heart failure is characterized by attenuated β‐adrenoceptor‐mediated positive inotropic effects, which is mostly attributed to a down‐regulation of β1‐adrenoceptors and uncoupling of β2‐adrenoceptors (Brodde & Michel, 1999). However, emerging evidence points to a different effect of β3‐adrenoceptors in heart failure. Increased expression of β3‐adrenoceptors at the mRNA and/or protein levels has been demonstrated in human heart failure (Moniotte, Kobzik, et al., 2001) and in rat (Ozakca et al., 2013; Sun et al., 2011; Zhao et al., 2013) and dog models of heart failure (Cheng et al., 2001; Table 1). β3‐adrenoceptor mRNA was also up‐regulated in neonatal rat cardiomyocytes upon treatment with noradrenaline (Germack & Dickenson, 2006; Watts et al., 2013). β3‐adrenoceptor protein, but not mRNA, was also increased in a rat model of exercise‐induced cardiac hypertrophy in the absence of heart failure (Barbier, Rannou‐Bekono, Marchais, Tanguy, & Carre, 2007). No β3‐adrenoceptor mRNA was detected in the heart of two patients with cardiomyopathy but no overt heart failure (Lindskog et al., 2015).
Table 1.
Regulation of β3‐adrenceptor mRNA and protein in disease models
| Species | mRNA | Protein | Reference |
|---|---|---|---|
| Senescence | |||
| Rat | n.r. | Up | Birenbaum et al. (2008) |
| Heart failure | |||
| Rat | No change | Up | Barbier, Rannou‐Bekono et al. (2007)* |
| Rat | n.r. | Up | Sun et al. (2011) |
| Rat | Up | n.r. | Zhao et al. (2013) |
| Rat | Up | n.r. | Ozakca et al. (2013) |
| Dog | Up | Up | Cheng et al., 2001 |
| Human | Up | Up | Moniotte, Vaerman, et al. (2001), Moniotte, Kobzik, et al. (2001) |
| Human | n.r. | Up | Napp et al. (2009) |
| Diabetes | |||
| Rat | Up | Up | Dinçer et al. (2001) |
| Rat | n.r. | Up | Amour et al. (2007) |
| Rat | n.r. | Up | Amour et al. (2008) |
| Rat | Up | n.r. | Kayki‐Mutlu et al. (2014) |
| Rat | n.r. | No change | Haley et al. (2015) |
| Rat | n.r. | No change | Jiang et al. (2015) |
| Hypothyroidism | |||
| Rat | Up | n.r. | Arioglu et al. (2010) |
Note: Note that data at the protein level are based on immunoblots, which are not always based on validated antibodies. n.r., not reported; *, animal model of exercise‐induced cardiac hypertrophy, not of heart failure. Note that the animal data in heart failure and in diabetes are based on several different models.
It remains unclear whether the enhanced expression of β3‐adrenoceptors in heart failure leads to a greater functionality of the receptor. Work in a rat model of isoprenaline‐induced heart failure using inhibition of L‐type Ca2+ channels as outcome parameter has suggested a greater function (Zhang et al., 2005). In contrast, using a similar model but with negative inotropic effects as an outcome parameter, it was found the response to BRL 37,344 was attenuated in heart failure; this attenuation was abolished by concomitant treatment with nebivolol but not with metoprolol (Ozakca et al., 2013). Nebivolol, a β1‐adrenoceptor antagonist with alleged β3‐adrenoceptor agonist properties, compared to the β1‐adrenoceptor antagonist metoprolol, also improved cardiac parameters such as coronary flow, left ventricular end diastolic pressure, and left ventricular weight to body weight ratio. An attenuated negative inotropic effect of BRL 37,344 was also found in failing human ventricular tissue, which was attributed to reduced expression of eNOS (Moniotte, Kobzik, et al., 2001). However, the decrease in β3‐adrenoceptor‐mediated negative inotropic effect was less compared to the reduction of β1‐ and β2‐adrenoceptor‐mediated positive effect in the failing heart, which results in an imbalance between the opposite inotropic effects. Interpretation of these findings is complicated by the finding that the inhibitory effects of β3‐adrenoceptor agonists on contractility and Ca2+ channels are blocked by NOS inhibitors or PTX; however, NOS expression is down‐regulated whereas that of PTX‐sensitive Gi proteins are up‐regulated in heart failure (Brodde & Michel, 1999). The balance of changes in the expression of both signalling molecules and β3‐adrenoceptors is potentially complex and difficult to predict. A further complicating factor is that β3‐adrenoceptor agonists can up‐regulate NOS in normal heart (Watts et al., 2013) and in heart failure (Niu et al., 2012).
From the above findings it is questionable whether stimulating β3‐adrenoceptors may be beneficial in heart failure. In a mouse model of heart failure induced by aortic constriction, a 3‐week treatment with BRL 37,344 prevented left ventricular dilatation and partially suppressed superoxide generation; this beneficial effect of BRL 37,344 was abolished in nNOS knockout mice (Niu et al., 2012). In that model, transgenic overexpression of β3‐adrenoceptors also had beneficial effects including a reduction in fibrosis; these were NO‐dependent (Hermida Pouleur et al., 2018). In a rat model of aortic constriction‐induced heart failure, treatment with BRL 37,344 decreased left ventricular end systolic pressure and rate of contraction/relaxation but increased the left atrial mass (Zhao et al., 2013); this leaves the question as to whether the beneficial effects of β3‐adrenoceptor stimulation in heart failure may at least partly occur secondary to or despite those on atrial remodelling. In a rat model based on chronic infusion of noradrenaline, BRL 37,344 attenuated many of the noradrenaline‐induced alterations, including hypertrophy, apoptosis, fibrosis, reduced Na+/K+ ATPase activity, and cardiac filling and rate of contraction and or relaxation, (Kayki‐Mutlu et al., 2018).
However, not all investigators found that a β3‐adrenoceptor agonist increased cardiac contractility in animals with heart failure. In a dog model of pacing‐induced heart failure, ex vivo experiments demonstrated further attenuation of Ca2+ transients and contractile responses upon exposure to BRL 37,344, implying that an up‐regulation of β3‐adrenoceptors in heart failure (Table 1) may be harmful and not beneficial (Cheng et al., 2001). In a subsequent study in this model, the investigators reported that stimulation of β3‐adrenoceptors by endogenous catecholamines was correlated with impaired left ventricular function (Morimoto et al., 2004). In support of this result, β3‐adrenoceptor knockout mice exhibited reduced expression and function of sarcoplasmic reticulum Ca2+ ATPase and phosholamban, leading to the proposal that β3‐adrenoceptors should be inhibited and not stimulated in heart failure (Ziskoven et al., 2006). Whether increased contractility is beneficial in heart failure is under discussion (see Section 4).
In conclusion, data from patients with heart failure and several animal models thereof have demonstrated an up‐regulation of cardiac β3‐adrenoceptors, although this is mostly based on total cardiac extracts and, therefore, does not necessarily allow conclusions on cardiomyocytes. However, it remains controversial whether this leads to increased β3‐adrenoceptor function. Moreover, it also remains controversial whether stimulation or inhibition of β3‐adrenoceptors may be beneficial in heart failure. Whether this controversy is related to differences in models being used or other factors remains to be determined; specifically, limited validity of some of the tools used to generate the data may contribute to the controversy. If it is related to specific animal models, it remains to be determined which of them is predictive for patients or a subset of them.
3.3. Diabetes
Studies in several, but not all animal models of diabetes, have found increased cardiac β3‐adrenoceptor expression at the mRNA and/or protein level (Table 1). Thus, an up‐regulation was found in rats treated with streptozotocin, a type 1 diabetes model, at the mRNA (Dinçer et al., 2001; Kayki‐Mutlu et al., 2014) and protein level (Amour et al., 2007; Amour et al., 2008; Dinçer et al., 2001). One of these studies also demonstrated that treatment with insulin restored β3‐adrenoceptor expression to normal levels (Dinçer et al., 2001). However, no up‐regulation of β3‐adrenoceptor protein was observed in this model by others (Haley et al., 2015) or in diabetic Zucker rats, a type 2 diabetes model (Jiang et al., 2015). Whether these differences are model‐related remains to be seen.
Diabetes has deleterious effects on the heart such as impaired contractility and relaxation. Decreased β‐adrenergic responsiveness is one of the most important features of the diabetic heart (Dinçer, Onay, Arı, Özçelikay, & Altan, 1998). Although the changes in inotropic responses induced by β1‐ and β2‐adrenoceptor stimulation have been well characterized in diabetic heart, β3‐adrenoceptor‐mediated relaxation has been hardly investigated. Among the few studies, it was found that a β3‐adrenoceptor‐mediated negative inotropic effect contributes to the attenuated positive inotropic effect induced by β‐adrenoceptor stimulation in diabetic rat heart (Amour et al., 2007). The same group later reported that β3‐adrenoceptors are not involved in the lusitropic effect of isoprenaline in diabetic rat heart (Amour et al., 2008). Of note, a down‐regulation of cardiac β1‐adrenoceptors in diabetes has been observed irrespective of whether the β3‐adrenoceptor was up‐regulated in the same animals, implying that the balance between positive inotropic effects by the former and negative inotropic effects by the latter may be shifted (Dinçer et al., 2001). Interestingly, the attenuated negative inotropic effect of BRL 37,344 in diabetic rats was restored upon insulin treatment (Arioglu‐Inan, Ozakca, Kayki‐Mutlu, Sepici‐Dincel, & Altan, 2013). Based on these findings, it has been proposed that augmented negative inotropic effects of β3‐adrenoceptor stimulation could be interpreted as a protective mechanism in the presence of sympathetic overactivation in the early stages of diabetes, but it could become harmful in later phases (Amour et al., 2007). In New Zealand white rabbits made hyperglycaemic by administration of an insulin receptor antagonist, treatment with CL 316,243 reduced oxidative stress and Na+/K+ ATPase function (Galougahi et al., 2015), raising the possibility that at least in some settings, β3‐adrenoceptor stimulation may be advantageous in diabetes.
3.4. Hypothyroidism
Thyroid hormones have important effects on cardiac contractility and relaxation. In hypothyroidism, diastolic function has been shown to be impaired as a result of reduced Ca2+ cycling (Kiss, Jakab, Kranias, & Edes, 1994). Despite increased β3‐adrenoceptor mRNA expression and increased expression of eNOS, the negative inotropic effects of BRL 37,344 were attenuated in hypothyroid rats (Arioglu, Guner, Ozakca, Altan, & Ozcelikay, 2010); whether these are mediated by β3‐adrenoceptors has not been assessed in that study.
4. β3‐ADRENOCEPTORS AS A THERAPEUTIC TARGET IN CARDIAC PATHOLOGIES
Whether β3‐adrenoceptor is present and physiologically relevant in the normal heart, has remained controversial in most species, particularly in humans (see Section 2). However, studies in various animal models and in patients have routinely reported an up‐regulation of β3‐adrenoceptor mRNA and/or protein (Table 1, Section 3). This raises the possibility that an increased expression of β3‐adrenoceptors may be a general response of the heart in pathophysiological settings. On a more pragmatic level, this opens up the possibility that β3‐adrenoceptor ligands may be useful in the treatment of cardiac disease, even in species where their expression is limited in the normal heart.
Based on the variable alterations in β3‐adrenoceptors observed in different cardiac pathologies and the various effects of β3‐adrenoceptor stimulation in such settings, it remains unclear whether agonism or antagonism of these receptors will be helpful in patients. Because it had been suggested that nebivolol is not only a β1‐adrenoceptor antagonist but also a β3‐adrenoceptor agonist (Rozec et al., 2009), several studies with nebivolol in cardiac pathologies have been interpreted as providing evidence of the benefits of β3‐adrenoceptor stimulation in animals (Ma et al., 2012) and patients (Flather et al., 2005). Although the vasodilating properties of nebivolol in some vascular beds are undisputed, mechanistic interpretation of the clinical trials is difficult because they do not allow the differentiation of the effects of β1‐adrenoceptor antagonism from those of possible β3‐adrenoceptor agonism. Moreover, studies with human β3‐adrenoceptor subtypes failed to detect agonist or antagonistic effects of nebivolol in concentrations compatible with those acting on β1‐adrenoceptors (Frazier et al., 2011).
More relevant data have been or are currently being obtained with the new β3‐adrenoceptor agonist mirabegron, that has been in clinical use for the treatment of overactive bladder syndrome since 2012 (Chapple, Cardozo, Nitti, Siddiqui, & Michel, 2014). Currently, there are three clinical studies investigating potential beneficial effects of mirabegron. One of them, the BEAT‐HF trial, has already been completed (Bundgaard et al., 2017). This double‐blind clinical trial has examined the effectiveness of mirabegron treatment versus placebo in 70 patients with NYHA class II–III heart failure. The treatment dose of mirabegron ranged between 25 and 150 mg (twice daily each). The aim of the trial was to determine safety and the beneficial effects of mirabegron on the heart in patients with heart failure. No significant change in left ventricular ejection fraction was observed after a 6‐month treatment with mirabegron. However, exploratory analysis has shown that LVEF was increased in mirabegron‐treated patients with a baseline ejection fraction lower than 40%. Mirabegron treatment did not alter blood pressure or heart rate compared to placebo.
Another clinical trial on mirabegron is Beta3_LVH, a randomized placebo‐controlled, double blind, multicentre trial on 296 patients (Pouleur et al., 2018). Patients with left ventricular hypertrophy are treated with 50 mg mirabegron (once daily) for 12 months. The study aimed to investigate the effects of mirabegron on left ventricle mass index and diastolic function in patients with left ventricular hypertrophy. This trial is expected to be completed in 2020.
SPHERE‐HF is also a randomized, placebo controlled, and multicentre trial which aims to determine the efficacy and safety of mirabegron in patients with pulmonary hypertension secondary to heart failure. It is conducted in 80 patients with a dose of mirabegron ranging between 50 mg (once daily) and 200 mg (once daily). It is estimated to be completed in 2019.
In conclusion, several clinical studies have recently been reported or are ongoing. Those performed in patients with heart failure are proof‐of‐concept or dose‐ranging studies, and the only well powered study has cardiac hypertrophy as its endpoint. While they will yield interesting insights, definitive trials will only be started once data from the dose‐ranging studies are in. All these trials are based on the idea that stimulating β3‐adrenoceptors could be beneficial in cardiac pathologies since stimulation of this subtype has been associated with antioxidant (Hermida Pouleur et al., 2018), antihypertrophic (Niu et al., 2012), and Na+‐K+‐pump activating effects (Bundgaard et al., 2010). If proven as a therapeutic modality, the combination of β3‐adrenoceptor agonists with other treatments enhancing the NO/cGMP pathways could have additional beneficial effects (Farah, Michel, & Balligand, 2018). Clinical studies based on the opposite premise, that is, that inhibition of β3‐adrenoceptors is beneficial in heart failure, are not ongoing to the best of our knowledge, possibly because no suitable candidate compound has been disclosed.
4.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017; Alexander, Striessnig et al., 2017).
CONFLICT OF INTEREST
E.A.I. and G.K.M. do not report a conflict of interest. M.C.M. is a consultant to Astellas and a consultant and shareholder of Velicept.
ACKNOWLEDGEMENTS
Work in the authors' labs has been supported in part by grants to E.A.I. from TUBITAK (Grant SBAG‐115S564) and Ankara University (Grants 15L0237005, 16L0237006, and 17L0237002) and to M.C.M. from Deutsche Forschungsgemeinschaft (Grant Mi 294/8‐1), Astellas, and Velicept.
Arioglu‐Inan E, Kayki‐Mutlu G, Michel MC. Cardiac β3‐adrenoceptors—A role in human pathophysiology? Br J Pharmacol. 2019;176:2482–2495. 10.1111/bph.14635
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 172, 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … Collaborators, C. G. T. P. (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … Collaborators, C. G. T. P. (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174(S1), S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … Collaborators, C. G. T. P. (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174(S1), S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amour, J. , Loyer, X. , Le Guen, M. , Mabrouk, N. , David, J.‐S. , Camors, E. , … Riou, B. (2007). Altered contractile response due to increased β3‐adrenoceptor stimulation in diabetic cardiomyopathy. The role of nitric oxide synthase 1‐derived nitric oxide. Anesthesiology, 107, 452–460. [DOI] [PubMed] [Google Scholar]
- Amour, J. , Loyer, X. , Michelet, P. , Birenbaum, A. , Riou, B. , & Heymes, C. (2008). Preservation of the positive lusitropic effect of β‐adrenoceptors stsimulation in diabetic cardiomyopathy. Anesthesia and Analgesia, 107, 1130–1138. [DOI] [PubMed] [Google Scholar]
- Arioglu, E. , Guner, S. , Ozakca, I. , Altan, V. M. , & Ozcelikay, A. T. (2010). The changes in β‐adrenoceptor‐mediated cardiac function in experimental hypothyroidism: The possible contribution of cardiac β3‐adrenoceptors. Molecular and Cellular Biochemistry, 335, 59–66. [DOI] [PubMed] [Google Scholar]
- Arioglu‐Inan, E. , Ozakca, I. , Kayki‐Mutlu, G. , Sepici‐Dincel, A. , & Altan, V. M. (2013). The role of insulin–thyroid hormone interaction on β‐adrenoceptor‐mediated cardiac responses. European Journal of Pharmacology, 718, 533–543. [DOI] [PubMed] [Google Scholar]
- Baker, J. G. (2010). The selectivity of ß‐adrenoceptor agonists at human ß1‐, ß2‐ and ß3‐adrenoceptors. British Journal of Pharmacology, 160, 1048–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier, J. , Mouas, C. , Rannou‐Bekono, F. , & Carré, F. (2007). Existence of β3‐adrenoceptors in rat heart: Functional implications. Clinical and Experimental Pharmacology & Physiology, 34, 796–798. [DOI] [PubMed] [Google Scholar]
- Barbier, J. , Rannou‐Bekono, F. , Marchais, J. , Tanguy, S. , & Carre, F. (2007). Alterations of β3‐adrenoceptors expression and their myocardial functional effects in physiological model of chronic exercise‐induced cardiac hypertrophy. Molecular and Cellular Biochemistry, 300, 69–75. [DOI] [PubMed] [Google Scholar]
- Berkowitz, D. E. , Nardone, N. A. , Smiley, R. M. , Price, D. T. , Kreutter, D. K. , Fremeau, R. T. , … Schwinn, D. A. (1995). Distribution of ß3‐adrenoceptor mRNA in human tissues. European Journal of Pharmacology, 289, 223–228. [DOI] [PubMed] [Google Scholar]
- Birenbaum, A. , Tesse, A. , Loyer, X. , Michelet, P. , Andriantsitohaina, R. , Heymes, C. , … Amour, J. (2008). Involvement of β3‐adrenoceptor in altered β‐adrenergic response in senescent heart. Role of nitric oxide synthase 1‐derived nitric oxide. Anesthesiology, 109, 1045–1053. [DOI] [PubMed] [Google Scholar]
- Bosch, R. F. , Schneck, A. C. , Kiehn, J. , Zhang, W. , Hambrock, A. , Eigenberger, B. W. , … Kuehlkamp, V. (2002). ß3‐Adrenergic regulation of an ion channel in the heart—Inhibition of the slow delayed rectifyer potassium channel IKs in guinea pig ventricular myocytes. Cardiovascular Research, 56, 393–403. [DOI] [PubMed] [Google Scholar]
- Brixius, K. , Bloch, W. , Pott, C. , Napp, A. , Krahwinkel, A. , Ziskoven, C. , … Schwinger, R. H. (2004). Mechanisms of ß3‐adrenoceptor‐induced eNOS activation in right atrial and left ventricular human myocardium. British Journal of Pharmacology, 143, 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodde, O. E. , & Michel, M. C. (1999). Adrenergic and muscarinic receptors in the human heart. Pharmacological Reviews, 51, 651–689. [PubMed] [Google Scholar]
- Brown, L. , Deighton, N. M. , Bals, S. , Söhlmann, W. , Zerkowski, H. R. , Michel, M. C. , & Brodde, O. E. (1992). Spare receptors for ß‐adrenoceptor‐mediated positive inotropic effects of catecholamines in the human heart. Journal of Cardiovascular Pharmacology, 19, 222–232. [DOI] [PubMed] [Google Scholar]
- Bundgaard, H. , Axelsson, A. , Hartvig Thomsen, J. , Sørgaard, M. , Kofoed Klaus, F. , Hasselbalch, R. , … Rasmussen, H. H. (2017). The first‐in‐man randomized trial of a β3 adrenoceptor agonist in chronic heart failure: The BEAT‐HF trial. European Journal of Heart Failure, 19, 566–575. [DOI] [PubMed] [Google Scholar]
- Bundgaard, H. , Liu, C. C. , Garcia, A. , Hamilton, E. J. , Huang, Y. , Chia, K. K. M. , … Rasmussen, H. H. (2010). ß3 Adrenergic stimulation of the cardiac Na+‐K+ pump by reversal of an inhibitory oxidative modification. Circulation, 122, 1699–1708. [DOI] [PubMed] [Google Scholar]
- Bylund, D. B. , Eikenberg, D. C. , Hieble, J. P. , Langer, S. Z. , Lefkowitz, R. J. , Minneman, K. P. , … Trendelenburg, U. (1994). IV. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacological Reviews, 46, 121–136. [PubMed] [Google Scholar]
- Cernecka, H. , Ochodnicky, P. , Lamers, W. H. , & Michel, M. C. (2012). Specificity evaluation of antibodies against human ß3‐adrenoceptors. Naunyn‐Schmiedeberg's Archives of Pharmacology, 385, 875–882. [DOI] [PubMed] [Google Scholar]
- Cernecka, H. , Pradidarcheep, W. , Lamers, W. H. , Schmidt, M. , & Michel, M. C. (2014). Rat ß3‐adrenoceptor protein expression: Antibody validation and distribution in rat gastrointestinal and urogenital tissues. Naunyn‐Schmiedeberg's Archives of Pharmacology, 387, 1117–1127. [DOI] [PubMed] [Google Scholar]
- Cernecka, H. , Sand, C. , & Michel, M. C. (2014). The odd sibling: Features of ß3‐adrenoceptor pharmacology. Molecular Pharmacology, 86, 479–484. [DOI] [PubMed] [Google Scholar]
- Chamberlain, P. D. , Jennings, K. H. , Paul, F. , Cordell, J. , Holmes, S. D. , Park, J. , … Murphy, G. J. (1999). The tissue distribution of the human ß3‐adrenoceptor studied using a monoclonal antibody: Direct evidence of the ß3‐adrenoceptor in human adipose tissue, atrium and skeletal muscle. International Journal of Obesity and Related Metabolic Disorders, 23, 1057–1065. [DOI] [PubMed] [Google Scholar]
- Chapple, C. R. , Cardozo, L. , Nitti, V. W. , Siddiqui, E. , & Michel, M. C. (2014). Mirabegron in overactive bladder: A review of efficacy, safety, and tolerability. Neurourology and Urodynamics, 33, 17–30. [DOI] [PubMed] [Google Scholar]
- Chapple, C. R. , Dvorak, V. , Radziszewski, P. , van Kerrebroeck, P. , Wyndaele, J. J. , Bosman, B. , … Dragon Investigator Group (2013). A phase II dose‐ranging study of mirabegron in patients with overactive bladder. International Urogynecoloy Journal, 24, 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H. J. , Zhang, Z. S. , Onishi, K. , Ukai, T. , Sane, D. C. , & Cheng, C. P. (2001). Upregulation of functional β3‐adrenergic receptor in the failing canine myocardium. Circulation Research, 89, 599–606. [DOI] [PubMed] [Google Scholar]
- Christ, T. , Molenaar, P. , Klenowski, P. M. , Ravens, U. , & Kaumann, A. J. (2011). Human atrial ß1L‐adrenoceptor but not ß3‐adrenoceptor activation increases force and Ca2+ current at physiological temperature. British Journal of Pharmacology, 162, 823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M. L. , Bloomquist, W. , Kriauciunas, A. , Shuker, A. , & Calligaro, D. (1999). Aryl propanolamines: Comparison of activity at human ß3 receptors, rat ß3 receptors and rat atrial receptors mediating tachycardia. British Journal of Pharmacology, 126, 1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis, R. , Arch, J. R. S. , Petroni, M. L. , Ferrari, D. , Cinti, S. , & Stock, M. J. (2002). Immunohistochemical identification of the ß3‐adrenoceptor in intact human adipocytes and ventricular myocardium: Effect of obesity and treatment with ephedrine and caffeine. International Journal of Obesity, 26, 1442–1450. [DOI] [PubMed] [Google Scholar]
- Devic, E. , Xiang, Y. , Gould, D. , & Kobilka, B. (2001). ß‐Adrenergic receptor subtype‐specific signaling in cardiac myocytes from ß1 and ß2 adrenoceptor knockout mice. Molecular Pharmacology, 60, 577–583. [PubMed] [Google Scholar]
- Dinçer, Ü. D. , Bidasee, K. R. , Güner, Ş. , Tay, A. , Özçelikay, A. T. , & Altan, V. M. (2001). The effect of diabetes on expression of β1‐, β2‐, and β3‐adrenoreceptors in rat hearts. Diabetes, 50, 455–461. [DOI] [PubMed] [Google Scholar]
- Dinçer, Ü. D. , Onay, A. , Arı, N. , Özçelikay, A. T. , & Altan, V. M. (1998). The effects of diabetes on β‐adrenoceptor mediated responsiveness of human and rat atria. Diabetes Research and Clinical Practice, 40, 113–122. [DOI] [PubMed] [Google Scholar]
- Emorine, L. J. , Marullo, S. , Briden‐sutren, M. M. , Patey, G. , Tate, K. , Delavier‐Klutchko, C. , & Strosberg, A. D. (1989). Molecular characterization of the human ß3‐adrenergic receptor. Science, 245, 1118–1121. [DOI] [PubMed] [Google Scholar]
- Evans, B. A. , Papaioannou, M. , Anastasopoulos, F. , & Summers, R. J. (1998). Differential regulation of β3‐adrenoceptors in gut and adipose tissue of genetically obese (ob/ob) C57BL/6J‐mice. British Journal of Pharmacology, 124, 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, B. A. , Papaioannou, M. , Hamilton, S. , & Summers, R. J. (1999). Alternative splicing generates two isoforms of the ß3‐adrenoceptor which are differentially expressed in mouse tissues. British Journal of Pharmacology, 127, 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah, C. , Michel, L. Y. M. , & Balligand, J.‐L. (2018). Nitric oxide signalling in cardiovascular health and disease. Nature Reviews Cardiology, 15, 292. [DOI] [PubMed] [Google Scholar]
- Flather, M. D. , Shibata, M. C. , Coats, A. J. S. , Van Veldhuisen, D. J. , Parkhomenko, A. , Borbola, J. , … SENIORS Investigators (2005). Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). European Heart Journal, 26, 215–225. [DOI] [PubMed] [Google Scholar]
- Frazier, E. P. , Michel‐Reher, M. B. , van Loenen, P. , Sand, C. , Schneider, T. , Peters, S. L. M. , … Michela, M. C. (2011). Lack of evidence that nebivolol is a ß3‐adrenoceptor agonist. European Journal of Pharmacology, 654, 86–91. [DOI] [PubMed] [Google Scholar]
- Frazier, E. P. , Schneider, T. , & Michel, M. C. (2006). Effects of gender, age and hypertension on ß‐adrenergic receptor function in rat urinary bladder. Naunyn‐Schmiedeberg's Archives of Pharmacology, 373, 300–309. [DOI] [PubMed] [Google Scholar]
- Galougahi, K. K. , Liu, C.‐C. , Garcia, A. , Fry, N. A. , Hamilton, E. J. , Figtree, G. A. , … Rasmussen, H. H. (2015). β3‐Adrenoceptor activation relieves oxidative inhibition of the cardiac Na+‐K+ pump in hyperglycemia induced by insulin receptor blockade. American Journal of Physiology ‐ Cell Physiology, 309, C286–C295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galougahi, K. K. , Liu, C. C. , Garcia, A. , Gentile, C. , Fry, N. A. , Hamilton, E. J. , … Figtree, G. A. (2016). β3‐Adrenergic stimulation restores nitric oxide/redox balance and enhances endothelial function in hyperglycemia. Journal of the American Heart Association, 5, e002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, C. , Langin, D. , & Balligand, J. L. (2000). ß3‐Adrenoceptors in the cardiovascular system. Trends in Pharmacological Sciences, 21, 426–431. [DOI] [PubMed] [Google Scholar]
- Gauthier, C. , Leblais, V. , Kobzik, L. , Trochu, J. N. , Khandoudi, N. , Bril, A. , & Le Marec, H. (1998). The negative inotropic effect of β3‐adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. The Journal of Clinical Investigation, 102, 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, C. , Tavernier, G. , Charpentier, F. , Langin, D. , & Le Marec, H. (1996). Functional ß3‐adrenoceptor in the human heart. The Journal of Clinical Investigation, 98, 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, C. , Tavernier, G. , Trochu, J. N. , Leblais, V. , Laurent, K. , Langin, D. , & Le Marec, H. (1999). Interspecies differences in the cardiac negative inotropic effects of ß3‐adrenoceptor agonists. The Journal of Pharmacology and Experimental Therapeutics, 290, 687–693. [PubMed] [Google Scholar]
- Germack, R. , & Dickenson, J. M. (2006). Induction of ß3‐adrenergic receptor functional expression following chronic stimulation with noradrenaline in neonatal rat cardiomyocytes. The Journal of Pharmacology and Experimental Therapeutics, 316, 392–402. [DOI] [PubMed] [Google Scholar]
- Guillaume, J. L. , Petitjean, F. , Haasemann, M. , Bianchi, C. , Eshdat, Y. , & Strosberg, A. D. (1994). Antibodies for the immunochemistry of the human ß3‐adrenergic receptor. European Journal of Biochemistry, 224, 761–770. [DOI] [PubMed] [Google Scholar]
- Guimaraes, S. , & Moura, D. (2001). Vascular adrenoceptors: An update. Pharmacological Reviews, 53, 319–356. [PubMed] [Google Scholar]
- Haley, J. M. , Thackeray, J. T. , Kolajova, M. , Thorn, S. L. , & DaSilva, J. N. (2015). Insulin therapy normalizes reduced myocardial β‐adrenoceptors at both the onset and after sustained hyperglycemia in diabetic rats. Life Sciences, 132, 101–107. [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. E. (1997). Lack of β‐3 adrenoceptor modulation of contractile function in human ventricular myocytes. Circulation, 96(Suppl), 53‐I. [Google Scholar]
- Harms, H. H. , Zaagsma, J. , & van der Wal, B. (1974). β‐Adrenoceptor studies. III. On the β‐adrenoceptors in rat adipose tissue. European Journal of Pharmacology, 25, 87–97. [DOI] [PubMed] [Google Scholar]
- Hermida, N. , Michel, L. , Esfahani, H. , Dubois‐Deruy, E. , Hammond, J. , Bouzin, C. , … Balligand, J. L. (2018). Cardiac myocyte β3‐adrenergic receptors prevent myocardial fibrosis by modulating oxidant stress‐dependent paracrine signaling. European Heart Journal, 39, 888–898. [DOI] [PubMed] [Google Scholar]
- Heubach, J. F. , Rau, T. , Eschenhagen, T. , Ravens, U. , & Kaumann, A. J. (2002). Physiological antagonism between ventricular ß1‐adrenoceptors and α1‐adrenoceptors but no evidence for ß2‐ and ß3‐adrenoceptor function in murine heart. British Journal of Pharmacology, 136, 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, C. , Leitz, M. R. , Oberdorf‐Maass, S. , Lohse, M. J. , & Klotz, K. N. (2004). Comparative pharmacology of human ß‐adrenergic receptor subtypes—Characterization of stably transfected receptors in CHO cells. Naunyn‐Schmiedeberg's Archives of Pharmacology, 369, 151–159. [DOI] [PubMed] [Google Scholar]
- Hutchinson, D. S. , Sato, M. , Evans, B. A. , Christopoulos, A. , & Summers, R. J. (2005). Evidence for pleiotropic signaling at the mouse ß3‐adrenoceptor revealed by SR59230A [3‐(2‐ethylphenoxy)‐1‐[(1,S)‐1,2,3,4‐tetrahydronapth‐1‐ylamino]‐2S‐2‐propanol oxalate]. The Journal of Pharmacology and Experimental Therapeutics, 312, 1064–1074. [DOI] [PubMed] [Google Scholar]
- Ikezono, K. , Michel, M. C. , Zerkowski, H. R. , Beckeringh, J. J. , & Brodde, O. E. (1987). The role of cyclic AMP in the positive inotropic effect mediated by ß1‐ and ß2‐adrenoceptors in the isolated human right atrium. Naunyn‐Schmiedeberg's Archives of Pharmacology, 335, 561–566. [DOI] [PubMed] [Google Scholar]
- Jiang, C. , Carillion, A. , Na, N. , De Jong, A. , Feldman, S. , Lacorte, J. M. , … Amour, J. (2015). Modification of the β‐adrenoceptor pathway in Zucker obese and obese diabetic rat myocardium. Critical Care Medicine, 43, e241–e249. [DOI] [PubMed] [Google Scholar]
- Kaidoh, K. , Igawa, Y. , Takeda, H. , Yamazaki, Y. , Akahane, S. , Miyata, H. , … Andersson, K. E. (2002). Effects of selective ß2 and ß3‐adrenoceptor agonists in detrusor hyperreflexia in conscious cerebral infarcted rats. The Journal of Urology, 168, 1247–1252. [DOI] [PubMed] [Google Scholar]
- Kaumann, A. J. (1996). (−)‐CGP 12177‐induced increase of human atrial contraction through a putative third ß‐adrenoceptor. British Journal of Pharmacology, 117, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann, A. J. , & Molenaar, P. (1997). Modulation of human cardiac function through 4 ß‐adrenoceptor populations. Naunyn‐Schmiedeberg's Archives of Pharmacology, 355, 667–681. [DOI] [PubMed] [Google Scholar]
- Kaumann, A. J. , Preitner, F. , Sarsero, D. , Molenaar, P. , Revelli, J. P. , & Giacobino, J. P. (1998). (−)‐CGP 12177 causes cardiostimulation and binds to cardiac putative ß4‐adrenoceptors in wild‐type and ß3‐adrenoceptor knockout mice. Molecular Pharmacology, 53, 670–675. [DOI] [PubMed] [Google Scholar]
- Kayki‐Mutlu, G. , Arioglu Inan, E. , Karaomerlioglu, I. , Altan, V. M. , Yersal, N. , Korkusuz, P. , … Zaza, A. (2018). Role of the β3‐adrenergic receptor subtype in catecholamine‐induced myocardial remodeling. Molecular and Cellular Biochemistry, 446, 149–160. [DOI] [PubMed] [Google Scholar]
- Kayki‐Mutlu, G. , Arioglu Inan, E. , Ozakca, I. , Ozcelikay, A. T. , & Altan, V. M. (2014). β3‐Adrenoceptor‐mediated responses in diabetic rat heart. General Physiology and Biophysics, 33, 99–109. [DOI] [PubMed] [Google Scholar]
- Kiss, E. , Jakab, G. , Kranias, E. G. , & Edes, I. (1994). Thyroid hormone‐induced alterations in phospholamban protein expression. Regulatory effects on sarcoplasmic reticulum Ca2+ transport and myocardial relaxation. Circulation Research, 75, 245–251. [DOI] [PubMed] [Google Scholar]
- Kohout, T. A. , Takaoka, H. , McDonald, P. H. , Perry, S. J. , Mao, L. , Lefkowitz, R. J. , & Rockman, H. A. (2001). Augmentation of cardiac contractility mediated by the human ß3‐adrenergic receptor overexpressed in the hearts of transgenic mice. Circulation, 104, 2485–2491. [DOI] [PubMed] [Google Scholar]
- Krief, S. , Lönnqvist, F. , Raimbault, S. , Baude, B. , van Spronsen, A. , Arner, P. , … Emorine, L. J. (1993). Tissue distribution of β 3‐adrenergic receptor mRNA in man. The Journal of Clinical Investigation, 91, 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann, F. A. , Limberg, B. J. , Artim, D. E. , Shah, M. , Downs, T. R. , Contract, D. , … de Groat, W. C. (2009). Effects of ß3‐adrenergic receptor activation on rat urinary bladder hyperactivity induced by ovariectomy. The Journal of Pharmacology and Experimental Therapeutics, 330, 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, B. , Qing, T. , Zhu, J. , Wen, Z. , Yu, Y. , Fukumura, R. , … Shi, L. (2017). A comprehensive mouse transcriptomic BodyMap across 17 tissues by RNA‐seq. Scientific Reports, 7, 4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D. , Qu, Y. , Tao, L. , Liu, H. , Hu, A. , Gao, F. , … Sun, J. Z. (2006). Inhibition of iNOS protects the aging heart against b2‐adrenergic receptor stimulation‐induced cardiac dysfunction and myocardial ischemic injury. The Journal of Surgical Research, 131, 64–72. [DOI] [PubMed] [Google Scholar]
- Lindskog, C. , Linné, J. , Fagerberg, L. , Hallström, B. M. , Sundberg, C. J. , Lindholm, M. , … Uhlén, M. (2015). The human cardiac and skeletal muscle proteomes defined by transcriptomics and antibody‐based profiling. BMC Genomics, 16, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Gul, R. , Habibi, J. , Yang, M. , Pulakat, L. , Whaley‐Connell, A. , … Sowers, J. R. (2012). Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the transgenic (mRen2) rat. American Journal of Physiology ‐ Heart and Circulatory Physiology, 302, H2341–H2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, J. C. W. , Nishikawa, M. , Haddad, E. B. , Kwon, O. J. , Hirst, S. J. , Twort, C. H. C. , & Barnes, P. J. (1996). Localisation and expression of ß‐adrenoceptor subtype mRNAs in human lung. European Journal of Pharmacology, 302, 215–221. [DOI] [PubMed] [Google Scholar]
- Malinowska, B. , & Schlicker, E. (1996). Mediation of the positive chronotropic effect of CGP 12177 and cyanopindolol in the pithed rat by atypical ß‐adrenoceptors, different from ß3‐adrenoceptors. British Journal of Pharmacology, 117, 943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska, B. , & Schlicker, E. (1997). Further evidence for differences between cardiac atypical ß‐adrenoceptors and brown adiopose tissue ß3‐adrenoceptors in the pithed rat. British Journal of Pharmacology, 122, 1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, M. C. , & Gravas, S. (2016). Safety and tolerability of ß3‐adrenoceptor agonists in the treatment of overactive bladder syndrome—Insight from transcriptosome and experimental studies. Expert Opinion on Drug Safety, 15, 647–657. [DOI] [PubMed] [Google Scholar]
- Michel, M. C. , Harding, S. E. , & Bond, R. A. (2011). Are there functional ß3‐adrenoceptors in the human heart? British Journal of Pharmacology, 162, 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo, W. , Michel, M. C. , Lee, X. W. , Kaumann, A. J. , & Molenaar, P. (2017). The β3‐adrenoceptor agonist mirabegron increases human atrial force through β1‐adrenoceptors: An indirect mechanism? British Journal of Pharmacology, 174, 2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniotte, S. , Kobzik, L. , Feron, O. , Trochu, J. N. , Gauthier, C. , & Balligand, J. L. (2001). Upregulation of ß3‐adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation, 103, 1649–1655. [DOI] [PubMed] [Google Scholar]
- Moniotte, S. , Vaerman, J. L. , Kockx, M. M. , Larrouy, D. , Langin, D. , Noirhomme, P. , … Balligand, J. L. (2001). Real‐time RT‐PCR for the detection of β‐adrenoceptor messenger RNAs in small human endomyocardial biopsies. Journal of Molecular and Cellular Cardiology, 33, 2121–2133. [DOI] [PubMed] [Google Scholar]
- Montastruc, J. L. , Verwaerde, P. , Pelat, M. , Galitzky, J. , Langin, D. , Lafontan, M. , & Berlan, M. (1999). Peripheral cardiovascular actions of SR 58611 A, a β 3‐adrenoceptor agonist, in the dog: Lack of central effect. Fundamental & Clinical Pharmacology, 13, 180–186. [DOI] [PubMed] [Google Scholar]
- Mori, A. , Miwa, T. , Sakamoto, K. , Nakahara, T. , & Ishii, K. (2010). Pharmacological evidence for the presence of functional ß3‐adrenoceptors in rat retinal blood vessels. Naunyn‐Schmiedeberg's Archives of Pharmacology, 382, 119–126. [DOI] [PubMed] [Google Scholar]
- Morimoto, A. , Hasegawa, H. , Cheng, H.‐J. , Little, W. C. , & Cheng, C.‐P. (2004). Endogenous β3‐adrenoreceptor activation contributes to left ventricular and cardiomyocyte dysfunction in heart failure. American Journal of Physiology ‐ Heart and Circulatory Physiology, 286, H2425–H2433. [DOI] [PubMed] [Google Scholar]
- Murakami, M. , Tomiyama, Y. , Hayakawa, K. , Akahane, M. , Ajisawa, Y. , Park, Y. C. , … Kurita, T. (2000). Effects of ß‐adrenergic stimulation on acutely obstructed ureter in dogs. The Journal of Pharmacology and Experimental Therapeutics, 292, 67–75. [PubMed] [Google Scholar]
- Muzzin, P. , Revelli, J. P. , Kuhne, F. , Gocayne, J. D. , McCombie, W. R. , Venter, J. C. , … Fraser, C. M. (1991). An adipose tissue‐specific ß‐adrenergic receptor. Molecular cloning and down‐regulation in obesity. The Journal of Biological Chemistry, 266, 24053–24058. [PubMed] [Google Scholar]
- Nahmias, C. , Blin, N. , Elalouf, J. M. , Mattei, M. G. , Strosberg, A. D. , & Emorine, L. J. (1991). Molecular characterization of the mouse ß3‐adrenergic receptor: Relationship with the atypical receptor of adipocytes. The EMBO Journal, 10, 3721–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napp, A. , Brixius, K. , Pott, C. , Ziskoven, C. , Boelck, B. , Mehlhorn, U. , … Bloch, W. (2009). Effects of the ß3‐adrenergic agonist BRL 37344 on endothelial nitric oxide synthase phosphorylation and force of contraction in human failing myocardium. Journal of Cardiac Failure, 15, 57–67. [DOI] [PubMed] [Google Scholar]
- Niclauß, N. , Michel‐Reher, M. B. , Alewijnse, A. E. , & Michel, M. C. (2006). Comparison of three radioligands for the labelling of human ß‐adrenoceptor subtypes. Naunyn‐Schmiedeberg's Archives of Pharmacology, 374, 99–105. [DOI] [PubMed] [Google Scholar]
- Niu, X. , Watts, V. L. , Cingolani, O. H. , Sivakumaran, V. , Leyton‐Mange, J. S. , Ellis, C. L. , … Barouch, L. A. (2012). Cardioprotective effect of β‐3 adrenergic receptor agonism: Role of neuronal nitric oxide synthase. Journal of the American College of Cardiology, 59, 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostendorp, J. , & Kaumann, A. J. (2000). Pertussis toxin suppresses carbachol‐evoked cardiodepression but does not modify cardiostimulation mediated through ß1‐ and putative ß4‐adrenoceptors in mouse left atria: No evidence for ß2‐ and ß3‐adrenoceptor function. Naunyn‐Schmiedeberg's Archives of Pharmacology, 361, 134–145. [DOI] [PubMed] [Google Scholar]
- Oriowo, M. A. , Chapman, H. , Kirkham, D. M. , Sennitt, M. V. , Ruffolo, R. R. Jr. , & Cawthorne, M. A. (1996). The selectivity in vitro of the stereoisomers of the β‐3 adrenoceptor agonist BRL 37344. The Journal of Pharmacology and Experimental Therapeutics, 277, 22–27. [PubMed] [Google Scholar]
- Ozakca, I. , Arioglu‐Inan, E. , Esfahani, H. , Altan, V. M. , Balligand, J.‐L. , Kayki‐Mutlu, G. , & Ozcelikay, A. T. (2013). Nebivolol prevents desensitization of β‐adrenoceptor signaling and induction of cardiac hypertrophy in response to isoprenaline beyond β1‐adrenoceptor blockage. American Journal of Physiology ‐ Heart and Circulatory Physiology, 304, H1267–H1276. [DOI] [PubMed] [Google Scholar]
- Pelat, M. , Verwaerde, P. , Galitzky, J. , Lafontan, M. , Berlan, M. , Senard, J. M. , … Montastruc, J. L. (2003). High isoproterenol doses are required to activate ß3‐adrenoceptor‐mediated functions in dogs. The Journal of Pharmacology and Experimental Therapeutics, 304, 246–253. [DOI] [PubMed] [Google Scholar]
- Piech, A. , Dessy, C. , Havaux, X. , Feron, O. , & Balligand, J. L. (2003). Differential regulation of nitric oxide synthases and their allosteric regulators in heart and vessels of hypertensive rats. Cardiovascular Research, 57, 456–467. [DOI] [PubMed] [Google Scholar]
- Pott, C. , Brixius, K. , Bundkirchen, A. , Bölck, B. , Bloch, W. , Steinritz, D. , … Schwinger, R. H. (2003). The preferential ß3‐adrenoceptor agonist BRL 37344 increases force via ß1‐/ß2‐adrenoceptors and induces endothelial nitric oxide synthase via ß3‐adrenoceptors in human atrial myocardium. British Journal of Pharmacology, 138, 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouleur, A.‐C. , Anker, S. , Brito, D. , Brosteanu, O. , Hasenclever, D. , Casadei, B. , … Balligand, J. L. (2018). Rationale and design of a multicentre, randomized, placebo‐controlled trial of mirabegron, a β3‐adrenergic receptor agonist on left ventricular mass and diastolic function in patients with structural heart disease β3‐left ventricular hypertrophy (Beta3‐LVH). ESC Heart Failure, 5(5), 830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradidarcheep, W. , Stallen, J. , Labruyere, W. T. , Dabhoiwala, N. F. , Michel, M. C. , & Lamers, W. H. (2009). Lack of specificity of commercially available antisera against muscarinic and adrenergic receptors. Naunyn‐Schmiedeberg's Archives of Pharmacology, 379, 397–402. [DOI] [PubMed] [Google Scholar]
- Ranade, K. , Jorgenson, E. , Sheu, W. H. , Pei, D. , Hsiung, C. A. , Chiang, F. T. , … Risch, N. (2002). A polymorphism in the ß1‐adrenergic receptor is associated with resting heart rate. American Journal of Human Genetics, 70, 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, H. H. , Figtree, G. A. , Krum, H. , & Bundgaard, H. (2009). Use of ß3 adrenergic receptor agonists in the treatment of heart failure. Current Opinion in Investigational Drugs, 10, 955–962. [PubMed] [Google Scholar]
- Rautureau, Y. , Toumaniantz, G. , Serpillon, S. , Jourdon, P. , Trochu, J. N. , & Gauthier, C. (2002). β 3‐adrenoceptor in rat aorta: Molecular and biochemical characterization and signalling pathway. British Journal of Pharmacology, 137, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa, G. M. , Baccino, D. , Valbusa, A. , Scala, C. , Barra, F. , Brunelli, C. , & Ferrero, S. (2018). Cardiovascular effects of antimuscarinic agents and β3‐adrenergic receptor agonist for the treatment of overactive bladder. Expert Opinion on Drug Safety, 17, 487497. [DOI] [PubMed] [Google Scholar]
- Rozec, B. , Erfanian, M. , Laurent, K. , Trochu, J. N. , & Gauthier, C. (2009). Nebivolol, a vasodilating selective ß1‐blocker is a ß3‐adrenoceptor agonist in the nonfailing transplanted heart. Journal of the American College of Cardiology, 53, 1532–1538. [DOI] [PubMed] [Google Scholar]
- Rozec, B. , & Gauthier, C. (2006). ß3‐Adrenoceptors in the cardiovascular system: Putative roles in human pathologies. Pharmacology & Therapeutics, 111, 652–673. [DOI] [PubMed] [Google Scholar]
- Scarpace, P. J. , Matheny, M. , & Thümer, N. (1999). Differential down‐regulation of ß3‐adrenergic receptor mRNA and signal transduction by cold exposure in brown adipose tissue of young and senescent rats. Pflügers Archiv / European Journal of Physiology, 437, 479–483. [DOI] [PubMed] [Google Scholar]
- Schneider, T. , Fetscher, C. , & Michel, M. C. (2011). Human urinary bladder strip relaxation by the ß‐adrenoceptor agonist isoprenaline: Methodological considerations and effects of gender and age. Frontiers in Pharmacology, 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennitt, M. V. , Kaumann, A. J. , Molenaar, P. , Beeley, L. J. , Young, P. W. , Kelly, J. , … Arch, J. R. (1998). The contribution of classical (ß1/2‐) and atypical ß‐adrenoceptors to the stimulation of white adipocyte lipolysis and right atrial appendage contraction by novel ß3‐adreoceptor agonists of differing selectivities. The Journal of Pharmacology and Experimental Therapeutics, 285, 1084–1095. [PubMed] [Google Scholar]
- Shen, Y. T. , Cervoni, P. , Claus, T. , & Vatner, S. F. (1996). Differences in ß3‐adrenergic receptor cardiovascular regulation in conscious primates, rats and dogs. Journal of Pharmacology and Experimental Therapeutics, 278, 1435–1443. [PubMed] [Google Scholar]
- Shen, Y. T. , Zhang, H. , & Vatner, S. F. (1994). Peripheral vascular effects of β‐3 adrenergic receptor stimulation in conscious dogs. The Journal of Pharmacology and Experimental Therapeutics, 268, 466–473. [PubMed] [Google Scholar]
- Skeberdis, V. A. , Gendviliene, V. , Zablockaite, D. , Teinys, R. , Macianskiene, R. , Bogdelis, A. , … Fischmeister, R. (2008). ß3‐Adrenergic receptor activation increases human atrial tissue contractility and stimulates the L‐type Ca2+ current. The Journal of Clinical Investigation, 118, 3219–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosberg, A. D. (1997). Structure and function of the ß3‐adrenergic receptor. Anuual Review of Pharmacology and Toxicology, 37, 421–450. [DOI] [PubMed] [Google Scholar]
- Sun, J. , Fu, L. , Tang, X. , Han, Y. , Ma, D. , Cao, J. , … Ji, H. (2011). Testosterone modulation of cardiac ß‐adrenergic signals in a rat model of heart failure. General and Comparative Endocrinology, 172, 518–525. [DOI] [PubMed] [Google Scholar]
- Takeda, H. , Yamazaki, Y. , Igawa, Y. , Kaidoh, K. , Akahane, S. , Miyata, H. , … Andersson, K. E. (2002). Effects of ß3‐adrenoceptor stimulation on prostaglandin E2‐induced bladder hyperreactivity and on the cardiovascular system in conscious rats. Neurourology and Urodynamics, 21, 558–565. [DOI] [PubMed] [Google Scholar]
- Tavernier, G. , Galitzky, J. , Bousquet‐Melou, A. , Montastruc, J. L. , & Berlan, M. (1992). The positive chronotropic effect induced by BRL 37344 and CGP 12177, two β‐3 adrenergic agonists, does not involve cardiac β adrenoceptors but baroreflex mechanisms. The Journal of Pharmacology and Experimental Therapeutics, 263, 1083–1090. [PubMed] [Google Scholar]
- Tavernier, G. , Toumaniantz, G. , Erfanian, M. , Heymann, M.‐F. , Laurent, K. , Langin, D. , & Gauthier, C. (2003). β3‐Adrenergic stimulation produces a decrease of cardiac contractility ex vivo in mice overexpressing the human β3‐adrenergic receptor. Cardiovascular Research, 59, 288–296. [DOI] [PubMed] [Google Scholar]
- Uhlen, M. , Fagerberg, L. , Hallström, B. M. , Lindskog, C. , Oksvold, P. , Mardinoglu, A. , … Pontén, F. (2015). Tissue‐based map of the human proteome. Science, 347, 1260419. [DOI] [PubMed] [Google Scholar]
- van Gelderen, M. , Stölzel, M. , Meijer, J. , Kerbusch, V. , Collins, C. , & Korstanje, C. (2017). An exploratory study in healthy male subjects of the mechanism of mirabegron‐induced cardiovascular effects. The Journal of Clinical Pharmacology, 57, 1534–1544. [DOI] [PubMed] [Google Scholar]
- van Wieringen, J. P. , Michel‐Reher, M. B. , Hatanaka, T. , Ueshima, K. , & Michel, M. C. (2013). The new radioligand [3H]‐L 748,337 differentially labels human and rat ß3‐adrenoceptors. European Journal of Pharmacology, 720, 124–130. [DOI] [PubMed] [Google Scholar]
- Watts, V. L. , Sepulveda, F. M. , Cingolani, O. H. , Ho, A. S. , Niu, X. , Kim, R. , … Baroucha, L. A. (2013). Anti‐hypertrophic and anti‐oxidant effect of β3‐adrenergic stimulation in myocytes requires differential neuronal NOS phosphorylation. Journal of Molecular and Cellular Cardiology, 62, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, C. , Wilson, S. , Piercy, V. , Sennitt, M. V. , & Arch, J. R. S. (1984). The rat lipolytic β‐adrenoceptor: Studies using novel β‐adrenoceptor agonists. European Journal of Pharmacology, 100, 309–319. [DOI] [PubMed] [Google Scholar]
- Woo, A. Y. H. , Song, Y. , Xiao, R. P. , & Zhu, W. (2015). Biased ß2‐adrenoceptor signalling in heart failure: Pathophysiology and drug discovery. British Journal of Pharmacology, 172, 5444–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. S. , Cheng, H. J. , Onishi, K. , Ohte, N. , Wannenburg, T. , & Cheng, C. P. (2005). Enhanced inhibition of L‐type Ca2+ current by ß3‐adrenergic stimulation in failing rat heart. The Journal of Pharmacology and Experimental Therapeutics, 315, 1203–1211. [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Zeng, F. , Liu, J.‐B. , He, Y. , Li, B. , Jiang, Z.‐F. , … Wang, L. X. (2013). Upregulation of β3‐adrenergic receptor expression in the atrium of rats with chronic heart failure. Journal of Cardiovascular Pharmacology and Therapeutics, 18, 133–137. [DOI] [PubMed] [Google Scholar]
- Ziskoven, C. , Grafweg, S. , Bölck, B. , Wiesner, R. J. , Jimenez, M. , Giacobino, J.‐P. , … Brixius, K. (2006). Increased Ca2+ sensitivity and protein expression of SERCA 2a in situations of chronic β3‐adrenoceptor deficiency. Pflügers Archiv ‐ European Journal of Physiology, 453, 443. [DOI] [PubMed] [Google Scholar]


