Abstract
β3‐Adrenoceptor agonists are used in the treatment of overactive bladder syndrome. Although the relaxant response to adrenergic stimulation in human detrusor smooth muscle cells is mediated mainly via β3‐adrenoceptors, the plasma concentrations of the therapeutic dose of mirabegron, the only clinically approved β3‐adrenoceptor agonist, are considerably lower than the EC50 for causing direct relaxation of human detrusor, suggesting a mechanism of action other than direct relaxation of detrusor smooth muscle. However, the site and mechanism of action of β3‐adrenoceptor agonists in the bladder have not been firmly established. Postulated mechanisms include prejunctional suppression of ACh release from the parasympathetic nerves during the storage phase and inhibition of micro‐contractions through β3‐adrenoceptors on detrusor smooth muscle cells or suburothelial interstitial cells. Implications of possible desensitization of β3‐adrenoceptors in the bladder upon prolonged agonist exposure and possible causes of rarely observed cardiovascular effects of mirabegron are also discussed.
Linked Articles
This article is part of a themed section on Adrenoceptors—New Roles for Old Players. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.14/issuetoc
Abbreviations
- BOO
bladder outlet obstruction
- DO
detrusor overactivity
- ENT
equilibrative nucleoside transporter
- IC
interstitial cell
- NDO
neurogenic detrusor overactivity
- NVC
non‐voiding contraction
- OAB
overactive bladder syndrome
- SAA
single‐unit afferent activity.
1. INTRODUCTION
The presence of a third subtype of β‐adrenoceptor was proposed long ago, for example, in adipose tissue (Harms, Zaagsma, & Van der Wal, 1974), but its existence was only established unequivocally when a human gene encoding the β3‐adrenoceptor was cloned (Emorine et al., 1989). β3‐adrenoceptors exhibit a restricted expression pattern in humans (Michel & Gravas, 2016), with a somewhat broader but still restricted expression in rats (Roberts, Papaioannou, Evans, & Summers, 1999) and mice (Nahmias et al., 1991). A potential role of β3‐adrenoceptors in the treatment of obesity and type 2 diabetes had been proposed based on the stimulation of lipolysis and thermogenesis in rats (Arch et al., 1984), but corresponding studies in humans failed, presumably because of a lack of expression of β3‐adrenoceptors in human adipose tissue (Michel & Korstanje, 2016).
The relaxation of human urinary bladder by β‐adrenoceptor agonists and its inhibition by antagonists could not be explained by the known β1‐ and β2‐adrenoceptors in early studies (Nergardh, Boreus, & Naglo, 1977), but unequivocal evidence for the involvement of β3‐adrenoceptors in the regulation of human bladder smooth muscle tone only came much later (Igawa et al., 1998; Igawa et al., 1999; Takeda et al., 1999). This led to the identification of β3‐adrenoceptors as a target for the treatment of bladder disease (Michel & Korstanje, 2016).
β3‐adrenoceptor agonists are primarily used in the treatment of overactive bladder syndrome (OAB). OAB is a symptom complex characterized by urgency, usually associated with frequency and nocturia, with or without urgency incontinence, in the absence of any other local pathology (Abrams et al., 2002). It is a common chronic condition with a prevalence of 10–20% and has a substantial negative impact on the quality of life of patients. While β3‐adrenoceptor agonists have proven effective and safe in the treatment of OAB (Chapple, Cardozo, Nitti, Siddiqui, & Michel, 2014; Ohlstein, von Keitz, & Michel, 2012; Yoshida, Takeda, Gotoh, Nagai, & Kurose, 2018), several important questions remain for a sound understanding of their mechanism of action. Against this background, we first summarize current knowledge on the expression and function of β3‐adrenoceptors in the normal and diseased bladder and the characteristics of β3‐adrenoceptor agonists for OAB treatment. Thereafter, we discuss key unknowns in relation to the use of β3‐adrenoceptor agonists in human bladder pathology.
2. EXPRESSION OF β3‐ADRENOCEPTORS IN THE BLADDER
2.1. mRNA level
The urinary bladder expresses mRNA for all three β‐adrenoceptor subtypes (Michel & Vrydag, 2006). While all three subtypes have similar abundance in rat bladder (Barendrecht et al., 2009), it has been reported that the β3‐adrenoceptor accounts for more than 95% of all β‐adrenoceptor mRNA in the human detrusor (Nomiya & Yamaguchi, 2003). However, others reported a comparable expression of all three subtypes using whole bladder rather than only detrusor tissue (Uhlen et al., 2015). The localization of β3‐adrenoceptor mRNA in the human detrusor has been explored in in situ hybridization studies (Takeda et al., 1999). However, the predictive value of β3‐adrenoceptor mRNA for the presence of functional receptor protein remains uncertain.
2.2. Protein level
Although many antibodies have limited selectivity for β3‐adrenoceptors (Cernecka, Ochodnicky, Lamers, & Michel, 2012), some antibodies have been validated as selective (Chamberlain et al., 1999; De Matteis et al., 2002; Guillaume et al., 1994). While the latter antibodies are still not perfect, it has been proposed that a combination of two antibodies, one directed against the N‐terminus and one against the C‐terminus of the receptor, may be informative if they yield the same staining pattern (Cernecka et al., 2012). This approach has successfully been applied (Coelho, Antunes‐Lopes, Gillespie, & Cruz, 2017; Silva et al., 2017).
A recent immunohistochemistry study in the human bladder detected β3‐adrenoceptors in smooth muscle fibres and, to a lesser extent, in urothelium and suburothelium (Silva et al., 2017). In contrast, another immunohistochemical study demonstrated β3‐adrenoceptors colocalized with the vesicular ACh transporter and primarily in nerve fibres in the mucosa and muscular layers of the bladder but not in urothelium or smooth muscle; the cholinergic fibres expressing β3‐adrenoceptors were found mostly in the suburothelium (Coelho et al., 2017). These two studies have used the same approach of concomitant labelling with multiple antibodies targeting different epitopes in the β3‐adrenoceptor, even with the same antibodies. Earlier studies based on validated antibodies have reported β3‐adrenoceptor expression to a greater extent in urothelium than smooth muscle of the human bladder and also in suburothelial myofibroblast‐like cells, intramural ganglions, Schwann cells, and intramural nerves (Limberg et al., 2010). Thus, various investigators using similar approaches and antibodies have obtained at least in part different results regarding the localization of β3‐adrenoceptors in the human urinary bladder. The reasons for these divergent results are not fully clear. However, it should be noted that sensitivity and specificity of immunohistological staining depend not only on the antibody being used but also on other factors, including thickness of slices, fixation and denaturalization protocols (Jositsch et al., 2009), and type of microscopy. Therefore, it is possible that rather minor differences in experimental protocol may have led to major differences in staining pattern, making it difficult to determine which cell types within the urinary bladder β3‐adrenoceptor are expressed at the protein level (Okeke, Gravas, & Michel, 2017).
3. ROLES OF β3‐ADRENOCEPTORS IN THE BLADDER PHYSIOLOGY
3.1. Detrusor smooth muscle
Human detrusor smooth muscle expresses predominantly β3‐adrenoceptor mRNA (Nomiya & Yamaguchi, 2003). Numerous studies show that relaxation of human bladder smooth muscle is mediated predominantly, if not exclusively, by the β3‐adrenoceptor subtype; however, the situation is different in other species, for example, a mix of β2‐ and β3‐adrenoceptors is involved in rat detrusor, which can complicate the interpretation of animal data (Igawa & Michel, 2013; Michel & Vrydag, 2006). There is no consistent evidence that isoprenaline can cause greater maximum bladder relaxation than selective β2‐ or β3‐adrenoceptor agonists in any species. Potential reasons for this observation include the possibility that activation of one of the two receptor subtypes is sufficient for a maximum effect, indicating a possible redundancy of their effects. However, this is not applicable to the human bladder where the contribution of β2‐adrenoceptors to relaxation is negligible (Michel & Vrydag, 2006). Secondly, β2‐adrenoceptor agonists may act via β3‐adrenoceptors because of limited selectivity. Thus, it has been proposed that fenoterol may cause relaxation of rat bladder smooth muscle via β3‐adrenoceptors (Palea et al., 2012), but this has not been confirmed by other investigators. In addition, β‐adrenoceptor agonists not only cause direct smooth muscle relaxation but also counteract contraction by stimulation of muscarinic receptors (Ehlert et al., 2007; Klausner, Rourke, Miner, & Ratz, 2009). β3‐adrenoceptor agonists inhibited the contractile responses evoked by electrical field stimulation to a much greater extent than those evoked by exogenous application of ACh in isolated human detrusor smooth muscle strips (Rouget et al., 2014). Such additional action of β3‐adrenoceptor agonists may be supported by a recent histological demonstration that an abundance of β3‐adrenoceptor immunoreactivity was observed on ACh‐containing nerve fibres coursing the suburothelium and the detrusor of the human bladder (Coelho et al., 2017). Work in rat detrusor has shown that β‐adrenoceptor agonists are more potent and more effective against any contractile stimulus other than muscarinic agonists (Cernecka, Pradidarcheep, Lamers, Schmidt, & Michel, 2014; Michel & Sand, 2009), which is in line with observations from various other types of smooth muscle (Dale et al., 2014). This raises the possibility that β3‐adrenoceptor agonists can inhibit detrusor contractions induced by pathological stimuli such as bradykinin, but largely spare those involved in physiological voiding and mediated by muscarinic receptors.
3.2. Urothelium
The urothelium actively participates in sensory functions, expressing various receptors for neurotransmitters and releasing neurotransmitters in response to various stimuli (Sellers, Chess‐Williams, & Michel, 2018). At the mRNA level, all three β‐adrenoceptor subtypes are expressed in the urothelium (Ochodnicky et al., 2012; Otsuka, Shinbo, Matsumoto, Kurita, & Ozono, 2008; Tyagi, Thomas, Yoshimura, & Chancellor, 2009). Immunohistochemical studies suggest that β3‐adrenoceptors are more abundant in the urothelium as compared to detrusor but β2‐adrenoceptors appear more relevant for the regulation of urothelial function (Sellers et al., 2018). The cellular mechanisms of β3‐adrenoceptor‐mediated effects of the urothelium on bladder function are not fully clear but appear to involve the afferent pathways innervating the bladder via release of NO or an unidentified inhibitory factor (urothelial‐derived inhibitory factor) from the urothelium (Figure 1).
Figure 1.
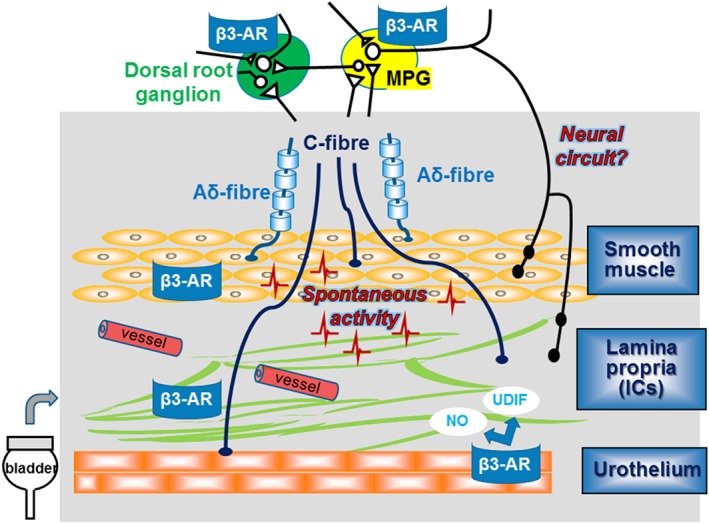
Expression of β3‐adrenoceptors (β3‐AR) and possible mechanisms that might be involved in the afferent pathway and modulation of spontaneous activity in the bladder
3.3. Suburothelial interstitial cells
One report, based on a poorly validated antibody, described no major difference in the staining intensity of β3‐adrenoceptor between the interstitial cells (ICs) and detrusor smooth muscle in the human urinary bladder, but both structures exhibited greater staining than the urothelium (Otsuka et al., 2013). The bladder suburothelium is abundant in sensory nerves and microvasculature, which may contribute to the maintenance of the function of the mucosa. Previous studies have demonstrated that the mucosa of guinea pig and pig bladders show spontaneous contractile activity, and one possible origin for these activities may be the suburothelial ICs (Biallosterski, van Koeveringe, van Kerrebroeck, Gillespie, & de Wachter, 2011; Hashitani, Takano, Fujita, Mitsui, & Suzuki, 2011; Heppner, Layne, Pearson, Sarkissian, & Nelson, 2011; Kushida & Fry, 2016; Moro, Leeds, & Chess‐Williams, 2012; Moro, Uchiyama, & Chess‐Williams, 2011). Mirabegron, a β3‐adrenoceptor agonist, reduced the frequency of non‐voiding contractions (NVCs), similar to spontaneous contractile activities, in rats with bladder outlet obstruction (BOO; Gillespie et al., 2012). These previous reports propose that β3‐adrenoceptor agonists can inhibit spontaneous contractile activities in the urinary bladder via β3‐adrenoceptors in ICs, and such spontaneous contractile activities may partly contribute to the development of bladder afferent hyperactivity (Aizawa et al., 2017). This would fit the theory of an urothelium‐associated sensory web linking sensory function as well as voiding function (Figure 1; Apodaca, 2004). However, in another recent study (Silva et al., 2017) very different findings were reported, that is, that β3‐adrenoceptors in human bladder were detected in smooth muscle fibres and, to a lesser extent, in the urothelium and suburothelium, and this may need further investigation.
3.4. Afferent nerves
Myelinated Aδ‐fibres are located primarily within the detrusor smooth muscle layer, whereas unmyelinated C‐fibres are more widespread and can be found not only in the detrusor but also in the lamina propria (including ICs), and often directly adjacent to the urothelial cells themselves (de Groat, 2004; Gabella & Davis, 1998; Ouslander, 2004; Vera & Nadelhaft, 2000). Previous studies indicated that β3‐adrenoceptors are expressed on bladder afferent nerves (Coelho et al., 2017) and dorsal root ganglion neurons (Kanai et al., 2012). Furthermore, afferent activities of both Aδ‐ and C‐fibres were intermittently enhanced by propagation of bladder myogenic micro‐contractions in BOO rats (Aizawa et al., 2017). Mirabegron can inhibit the mechanosensitive Aδ‐fibres, which may be related to suppression of the bladder micro‐contractions even in normal rats (Aizawa, Homma, & Igawa, 2012). Moreover, another study has identified a population of small‐diameter neurons in the major pelvic ganglion of the rat that express β3‐adrenoceptor immunoreactivity (Eastham, Stephenson, Korstanje, & Gillespie, 2015), suggesting a possibility of involvement of β3‐adrenoceptors on neural circuits in the regulation of afferent outflow and sensation (Figure 1).
3.5. Bladder vasculature
Blood vessels providing perfusion to the bladder possibly contribute to the regulation of bladder function. In a rat model of bladder hypoperfusion caused by experimental atherosclerosis, the relaxation responses of isolated detrusor strips to isoprenaline and salbutamol remained unchanged whereas those to BRL 37,344 were reported to be increased (Bayrak et al., 2015). In a different rat model of chronic bladder ischaemia induced by atherosclerosis, chronic treatment with mirabegron prevented bladder hyperactivity and collagen deposition in the bladder wall, suggesting β3‐adrenoceptor agonism may be a potential treatment target for chronic ischaemia‐related bladder dysfunction (Sawada et al., 2013).
3.6. In vivo studies
The in vivo effects of β3‐adrenoceptor agonists on bladder function have been studied mainly in rodents. β3‐adrenoceptor agonists increase bladder capacity without changes in micturition pressure and the residual volume during the voiding phase (Fujimura et al., 1999; Hicks et al., 2007; Kaidoh et al., 2002; Takeda et al., 2002; Woods, Carson, Norton, Sheldon, & Argentieri, 2001). Similarly, mirabegron decreased the frequency of rhythmic bladder contraction without suppressing its amplitude under isovolumetric conditions (Takasu et al., 2007). Thus, activation of β3‐adrenoceptors is associated with relaxation of the bladder during the storage phase of the micturition cycle without an effect on the voiding phase (Michel, Ochodnicky, Homma, & Igawa, 2011; Nitti et al., 2013). While not covered here in detail, β3‐adrenoceptors may also be involved in the regulation of other genitourinary tissues including the ureter (Matsumoto et al., 2013), prostate (Calmasini et al., 2015; Suzuki, Otsuka, Matsumoto, Furuse, & Ozono, 2016), and urethra (Alexandre et al., 2016).
β3‐adrenoceptors have been proposed to inhibit spontaneous myogenic contractions that may possibly generate afferent activity (Aizawa et al., 2017). Mirabegron can inhibit mechanosensitive bladder afferent activity, especially of myelinated Aδ‐fibres, which may be related to suppression of bladder myogenic spontaneous contractile activities at least under normal conditions (Aizawa et al., 2012). In addition, β3‐adrenoceptor agonists reduced bladder myogenic activity in rats with BOO (Gillespie et al., 2012; Hatanaka et al., 2013; Woods et al., 2001). This may lead to inhibition of abnormal bladder sensation related to enhanced myogenic spontaneous contractile activities in pathophysiological situations.
4. ROLES OF β3‐ADRENOCEPTORS IN PATHOPHYSIOLOGY OF OAB
Physiological detrusor contraction during the voiding phase is predominantly mediated by muscarinic M3 receptors in humans, but in OAB, additional mediators may contribute (Ouslander, 2004). The expression level of β‐adrenoceptor subtype mRNA was not significantly different in the human detrusor muscle with or without BOO condition (Nomiya & Yamaguchi, 2003), and mirabegron concentration‐dependently decreased carbachol‐induced detrusor smooth muscle tone in bladder samples obtained from healthy subjects and patients with BOO and BOO with detrusor overactivity (DO) to a similar extent (Svalo et al., 2013). β3‐adrenoceptor agonists may be more potent in decreasing nerve‐evoked ACh release than in producing direct relaxation of human bladder smooth muscle (D' Agostino, Maria Condino, & Calvi, 2015; Rouget et al., 2014). This is in line with the observed abundance of β3‐adrenoceptor immunoreactivity on ACh‐containing nerve fibres coursing the suburothelium and the detrusor of the human bladder, leading to the hypothesis that β3‐adrenoceptor agonists act, at least in part, through inhibition of ACh release from cholinergic, most probably parasympathetic, nerve terminals through a prejunctional mechanism (Coelho et al., 2017). According to this theory, β3‐adrenoceptor agonists would preferentially inhibit pathologically‐increased detrusor tone during bladder filling over physiological detrusor contraction during voiding and enabling them to inhibit an increased cholinergic tone in OAB. If so, combining them with a muscarinic antagonist would be promising therapeutic. While initial data support this idea (Abrams et al., 2017), dedicated clinical trials are required to confirm it.
5. CHARACTERISTICS OF β3‐ADRENOCEPTOR AGONISTS FOR OAB TREATMENT
Potency and efficacy at stimulating cAMP formation via β1‐, β2‐, and β3‐adrenoceptor for each of the compounds mentioned in this section is summarized in Table 1.
Table 1.
EC50 values for cAMP formation by β3‐adrenoceptor (AR) agonists in CHO cells transfected with human β‐adrenoceptor subtypes
| Drugs | EC50 | References | ||
|---|---|---|---|---|
| β1‐AR (nM) | β2‐AR (nM) | β3‐AR (nM) | ||
| Mirabegron (YM‐178) | >10,000 | >10,000 | 22.4 | Takasu et al. (2007) |
| Ritobegron (KUC‐7483) | 22,000 | 2,300 | 73 | Maruyama et al. (2012) |
| Solabegron (GW427353) | 3,980 | 1,260 | 123.98 | Uehling et al. (2006) |
| Vibegron (MK‐4618) | >20,000 | >20,000 | 1.1 | Edmondson et al. (2016) |
5.1. Mirabegron
Mirabegron (YM‐178) has higher affinity and greater intrinsic activity at β3‐ as compared to β1‐ and β2‐adrenoceptors (Table 1; Takasu et al., 2007). The efficacy and safety of mirabegron has been shown in numerous randomized placebo‐controlled studies in OAB (Chapple et al., 2014), leading to it being the first β3‐adrenoceptor agonist for the treatment of OAB.
5.2. Ritobegron
The selectivity of ritobegron (KUC‐7483) for β3‐adrenoceptors was 301 and 32 times higher than that for β1‐ and β2‐adrenoceptors, respectively (Table 1; Maruyama et al., 2012). In its first Phase III study, ritobegron did not significantly improve the mean number of micturitions per 24 hr compared to placebo. A long‐term safety and efficacy study was subsequently withdrawn, and ritobegron does not seem to have been developed further (Thiagamoorthy, Giarenis, & Cardozo, 2015).
5.3. Solabegron
Solabegron (GW427353) had an EC50 value of 1.9 nM in human bladder strips pre‐contracted with KCl, whereas isoprenaline had an EC50 value of 8.3 mM (Tyagi et al., 2009). In a Phase II multicentre, randomized, proof‐of‐concept trial in 258 women with wet OAB, the drug produced a statistically significant difference in percent change from baseline to Week 8 in incontinence episodes over 24 hr (primary outcome) when compared with placebo (P = 0.025) and was well tolerated (Ohlstein et al., 2012). An additional Phase II dose‐ranging study with a new formulation is currently ongoing.
5.4. Vibegron
Vibegron (MK‐4618) is a β3‐adrenoceptor agonist, potently activates human β3‐adrenoceptors with an EC50 value of 1.1 nM, and vibegron is also highly selective over β1‐ and β2‐adrenoceptors versus β3‐adrenoceptors across multiple species (Table 1). Vibegron did not show any stimulating or inhibitory effects on cytochrome P450 enzymes, suggesting a low risk of drug–drug interaction (Edmondson et al., 2016). A recent randomized placebo‐controlled Phase III study showed that the 12‐week treatment with vibegron (50 or 100 mg once daily) resulted in a significant improvement over the placebo in changes in the mean number of micturitions per day at Week 12 from baseline (primary endpoint) and changes from baselines in OAB symptom variables (daily episodes of urgency, urgency incontinence, incontinence, and nocturia, and voided volume/micturition) as the secondary endpoints. Vibegron also significantly improved quality of life, with high patient satisfaction. Incidence of drug‐related adverse events with vibegron 50 and 100 mg were similar to placebo, and less than imidafenacin, an anti‐muscarinic agent (Yoshida, Takeda, et al., 2018). In addition, a 1‐year, multicentre, open‐label, non‐controlled study confirmed that vibegron 50 and 100 mg have favourable safety profiles for the 52‐week treatment and improved OAB symptoms and quality of life, and a dose increase to 100 mg improved OAB symptoms without increasing adverse events in those patients who did not respond well to 50 mg vibegron (Yoshida, Kakizaki, Takahashi, Nagai, & Kurose, 2018).
6. KEY QUESTIONS FOR FUTURE RESEARCH
6.1. Does an endogenous β‐adrenoceptor agonist tone exist in the bladder, and if so, does it change in pathological conditions?
The presence of endogenous β‐adrenoceptor‐mediated tone to the bladder detrusor is controversial. Systemic sympathetic activity increases during the storage phase of the micturition cycle, and the noradrenaline released is believed to act on β‐adrenoceptors on the bladder wall to produce smooth muscle relaxation. This increases bladder compliance and enables continued low pressure filling of the bladder (reviewed in Fowler, Griffiths, & de Groat, 2008). Both nonselective β‐adrenoceptor and selective β3‐adrenoceptor agonism with mirabegron prolonged the inter‐void interval and increased bladder compliance while suppressing amplitude of NVCs but not their frequency (Sadananda, Drake, Paton, & Pickering, 2013). The β3‐adrenoceptor‐mediated effects promote storage without associated impairment of voiding function and are also seen in an acid‐sensitized bladder pathological model. The increase in the inter‐void interval correlates with the degree of increase in bladder compliance produced by β3‐adrenoceptor activation, but not with the reduction in NVC amplitude, nor with changed afferent sensitivity. Moreover, L 748,337, a selective β3‐adrenoceptor antagonist, shortened inter‐micturition interval and decreased bladder compliance, suggesting the presence of a basal β3‐adrenoceptor‐mediated sympathetic tone, or inverse agonistic activity. However, sympathetic innervation of the human detrusor is sparse (Gosling, Dixon, & Jen, 1999) and sympathectomy has no distinct effect on bladder filling (Andersson, 1986). Furthermore, a deficiency of dopamine β‐hydroxylase, the enzyme that converts dopamine to noradrenaline, does not lead to abnormal voiding in patients (Gary & Robertson, 1994). Additional investigations are necessary to better understand the role of endogenous noradrenaline, the sympathetic tone and its alteration in pathophysiological settings for urine storage in humans.
6.2. How can mirabegron relax human detrusor if plasma concentrations after therapeutic dosing do not exceed 100 nM but most studies find EC50 values in the low micromolar range?
Most studies using isolated bladder strips have reported a potency of mirabegron for causing relaxation in the low micromolar range (Igawa & Michel, 2013) but there are a few exceptions where an EC50 of about 100 nM has been reported (D' Agostino et al., 2015). However, plasma concentrations observed upon therapeutic doses of mirabegron typically do not exceed 100 nM (Krauwinkel et al., 2012). Based on this discrepancy, it has been proposed that the cellular target of β3‐adrenoceptor agonists in the treatment of OAB may not be the smooth muscle cell of the urinary bladder (Eastham et al., 2015) but rather a different cell type such as urothelium, ICs of Cajal, efferent, afferent nerves, the major pelvic ganglion, and/or blood vessels supplying the urinary bladder (Okeke et al., 2017). However, except for inhibition of ACh release from cholinergic nerves (D' Agostino et al., 2015; Silva et al., 2017), a submicromolar potency of mirabegron has not been shown in any of these alternative cell types. Inhibition of neuronal ACh release is an unlikely mechanisms of the therapeutic effects in OAB because neuronal ACh is implicated in physiological voiding but less so if at all in the DO typical for OAB (Michel & Chapple, 2009).
This raises the question whether the focus on bladder smooth muscle or some of our analytical approaches has been wrong. While we do not have a definitive answer to this question, several considerations apply. First, the potency of β‐adrenoceptor agonists to cause smooth muscle relaxation depends on the contractile agent against which they are tested; they are about 10‐fold less potent against muscarinic agonists than against other stimuli (Dale et al., 2014). Second, mirabegron is less potent against the rodent as compared to the human β3‐adrenoceptor (Igawa & Michel, 2013). Third, less than 50% of receptors need to be occupied to achieve a half‐maximal response for many agonist effects, a phenomenon called spare receptors or non‐linear stimulus effect coupling (Brown et al., 1992). Fourth, and perhaps most importantly, drug concentrations in plasma may underestimate those in the micro‐environment in which they are acting. Enrichment within target tissue has been shown for other drugs used in the treatment of lower urinary tract function (Korstanje, Krauwinkel, & van Doesum‐Wolters, 2011).
6.3. Do prejunctional β3‐adrenoceptors contribute to treatment effects on OAB?
Mirabegron had high potency for inhibiting the nerve‐evoked contraction and ACh release in human bladder preparation, with EC50 value of 123 and 129 nM respectively (D' Agostino et al., 2015). Inhibition of neuronal ACh release is so far the only cellular response to mirabegron in the human urinary bladder that has been consistently reported to occur at concentrations that are found in plasma of mirabegron‐treated subjects. The activation of β3‐adrenoceptors inhibits neurogenic contractions of both rat and human urinary bladders. β3‐adrenoceptor agonism also inhibits contractions induced by exogenously applied ACh. However, the effect is clearly less than that on neurogenic contractions (particularly in human), presuming that in addition to a direct effect on smooth muscle, activation of prejunctional β3‐adrenoceptors may inhibit ACh release from parasympathetic cholinergic nerve terminals. Supporting the inhibitory action of β3‐adrenoceptor agonists on nerve‐evoked ACh release, a recent immunohistochemistry study on human bladder tissue indicated that β3‐adrenoceptors are expressed only on cholinergic nerve fibres but are not present on urothelial or smooth muscle cells (Coelho et al., 2017). However, another study showed a controversial finding that β3‐adrenoceptors are diffusely distributed among detrusor smooth muscle cells but are not present on cholinergic nerve fibres, while adenosine A1 receptors are predominantly expressed on cholinergic nerve fibres (Silva et al., 2017). Indeed, both isoprenaline and mirabegron decreased ACh release induced by electrical field stimulation in the human detrusor preparation, which was prevented by β3‐adrenoceptor antagonists and DPCPX, an A1 receptor antagonist (Silva et al., 2017). Because β3‐adrenoceptors, similar to other β‐adrenoceptor subtypes, couple to stimulation of cAMP formation and cAMP is degraded to adenosine, Silva et al. tested whether adenosine may mediate the inhibition of ACh release observed with β3‐adrenoceptor agonists. In fact, isoprenaline and mirabegron increased extracellular adenosine concentrations in the detrusor strips. Antagonism of A1 receptors by DPCPX or blockade of the equilibrative nucleoside transporters (ENT) with dipyridamole prevented inhibition of ACh release, suggesting that it did not necessarily occur via a β3‐adrenoceptor located in the nerve ending but rather indirectly by intermediate formation of adenosine and subsequent activation of inhibitory A1 receptors. The authors demonstrated positive ENT1 and ENT2 immunoreactivities in human detrusor strips. These findings suggest that β3‐adrenoceptor agonists, including therapeutically‐achieved concentrations of mirabegron, can inhibit neuronal ACh release in human detrusor. They support a hypothesis that (indirect) inhibition of ACh release may be the mechanism for detrusor smooth muscle relaxation. However, it is not yet fully clear whether such inhibition of ACh release indeed occurs exclusively indirectly via adenosine formation and A1 receptor activation or whether it may also involve a neuronally‐expressed β3‐adrenoceptor. However, translating the cellular activity of β3‐adrenoceptor agonists to tissue and whole‐body systems is not straightforward and can often be misleading (Figure 2).
Figure 2.
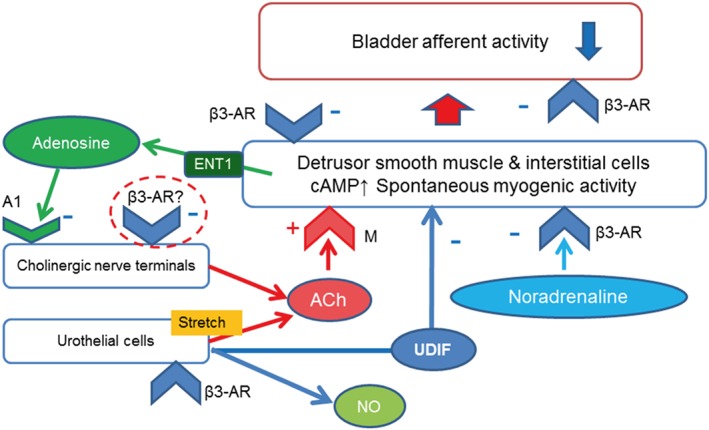
Hypothetic mechanism of mirabgron's inhibitory action on ACh release through the A1 receptors
6.4. Do β3‐adrenoceptor agonists inhibit bladder mechanosensitive afferent activity, and if so, how do they act?
Several studies have reported that detrusor smooth muscle show spontaneous contractile activity. In women with overactive bladder, these localized contractile activities increase during occurrence of urinary urgency without increasing intravesical pressure (Drake, Harvey, Gillespie, & Van Duyl, 2005). Sadananda et al. demonstrated in in vivo cystometry in the decerebrated rat model that intravesical administration of the selective β3‐adrenoceptor agonist mirabegron increased inter‐voiding intervals and compliance and decreased the amplitude of NVCs (Sadananda et al., 2013).
Studies in which bladder mechanosensitive single‐unit afferent activities (SAAs) were monitored in rats revealed that the afferent activities of both Aδ‐ and C‐fibres decreased after mirabegron administration in a dose‐dependent manner, which was more marked for Aδ‐fibres than C‐fibres; moreover, the inhibition of afferent activities appeared to synchronize with the decrease in fluctuation on bladder pressure (micro‐contractions), whereas bladder compliance did not change significantly from the baseline value with the mirabegron treatment at the doses used (Aizawa et al., 2012). In order to determine the relation of afferent activities with micro‐contractions and the effect of mirabegron on them, the authors further investigated these in an isovolumetric condition and confirmed the suppression of micro‐contractions concomitantly with Aδ‐fibre activity but not C‐fibre activity with 0.3‐mg·kg−1 mirabegron (Aizawa, Homma, & Igawa, 2015). I.v. administration of another β3‐adrenoceptor agonist, CL316,243 (10 μg·kg−1), similarly decreased Aδ‐fibre, but not C‐fibre, activities in response to bladder filling with saline. Intravesical instillation of PGE2 significantly increased C‐ but not Aδ‐fibre activities. The PGE2‐induced increase in C‐fibre activities was inhibited by pretreatment with CL316,243 (Aizawa, Igawa, Nishizawa, & Wyndaele, 2010). A recent study with similar monitoring bladder mechanosensitive SAAs in rats with BOO showed a higher number of micro‐contractions and lower SAAs of Aδ‐fibres, but SAAs of both Aδ‐ and C‐fibres were intermittently enhanced by micro‐contractions. These pathophysiological findings may contribute to the development of OAB associated with BOO (Aizawa et al., 2017).
Taken all together, the possible mechanisms of the inhibition of urgency induced by the β3‐adrenoceptor agonist may include (a) relaxing detrusor muscle‐decreasing bladder tone, (b) inhibiting micro‐contractions/Aδ‐fibre activity, and (c) inhibiting urotheliogenic afferent activation‐C‐fibre activity (Figure 3).
Figure 3.
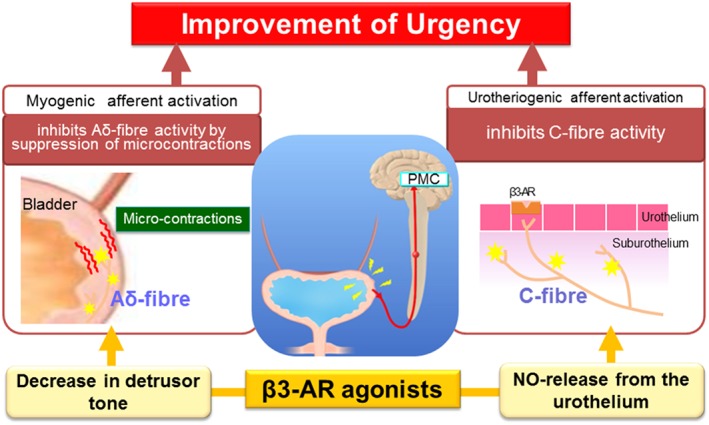
Hypothetic mechanisms involved in urgency improvement by β3‐adrenoceptor agonists
6.5. Do β3‐adrenoceptor agonists inhibit neurogenic detrusor overactivity?
While several β3‐adrenoceptor agonists appear effective in the treatment of OAB (Chapple et al., 2014; Ohlstein et al., 2012; Yoshida, Takeda, et al., 2018), only limited information, largely based on animal models, is available for neurogenic DO (NDO). An early study used the experimental β3‐adrenoceptor agonist FK175 in a rat model of DO based on ibotenic acid‐induced brain lesions (Fujimura et al., 1999). Acute administration of FK175 dose‐dependently increased bladder capacity without major changes in micturition or threshold pressure. In DO induced by cerebral infarction in rats, acute administration of the experimental β3‐adrenoceptor agonist CL316,243 also increased bladder capacity without changing voiding pressure or post‐voiding residual volume (Kaidoh et al., 2002). In contrast, the β2‐adrenoceptor agonist procaterol increased bladder capacity and residual volume but did not change voiding pressure. While CL316,243 had only minimal effects on cardiovascular function, procaterol lowered blood pressure and increased heart rate. Two studies have explored effects of β3‐adrenoceptor agonists in rat models of spinal cord injury. Acute administration of CL316,243 similarly increased inter‐contraction intervals in conscious sham and spinal cord injury rats and decreased micturition frequency without affecting amplitude of micturition (Beauval et al., 2015); moreover, CL316,243 reduced the frequency of NVCs in spinal cord injury rats by about half without major effects on amplitude of contractions. Others have explored effects of a 2‐ to 4‐week treatment with oxybutynin, mirabegron, and their combination in spinal cord injury rats (Wada et al., 2017). Both monotherapies tended to decrease NVCs with statistical significance being reached with the combination treatment. Combination treatment also improved bladder compliance and increased inter‐contraction intervals, voided volume, and bladder capacity.
There are few clinical studies on the efficacy of mirabegron for either NDO or low‐compliance bladder. Wöllner and Pannek recently reported that 15 patients with NDO treated with mirabegron for a period of at least 6 weeks showed a significant reduction in the frequency of bladder evacuation per 24 hr (8.1 vs. 6.4, P = 0.003) and of incontinence episodes per 24 hr (2.9 vs. 1.3, P = 0.027; Wollner & Pannek, 2016). Furthermore, urodynamic studies revealed improvements in bladder capacity (from 365 to 419 ml), compliance (from 28 to 45 ml·cmH2O−1), and detrusor pressure during storage phase (45.8 vs. 30 cmH2O). At follow‐up, 9/15 patients were satisfied with the therapy.
Wada et al. retrospectively examined the efficacy of combination therapy with mirabegron in seven patients (five men and two women) with NDO or low‐compliance bladder (<10 ml·cmH2O−1) refractory to anticholinergic treatment (Wada et al., 2015). Underlying diseases were spinal cord injury in three patients, spina bifida in two, spinal cord infarction in one, and post‐radical hysterectomy in one. After mirabegron, urinary incontinence was improved in all patients. G3 bladder deformity was improved to G2 and G1 in one patient each, and vesicoureteral reflux disappeared in all three patients. DO disappeared in two of the five patients, and bladder compliance was improved in all four patients with low‐compliance bladder.
Due to the limited number of patients and the retrospective nature of these studies, prospective, placebo‐controlled studies are required to confirm the beneficial effects of mirabegron on NDO suggested by these studies.
6.6. Do β3‐adrenoceptor agonists ameliorate poorly compliant detrusor?
Other than DO, a poorly compliant detrusor can be a cause of OAB‐like symptoms. There is only one clinical study available focusing on the effect of mirabegron on poor compliant detrusor. Kamei et al. investigated video‐urodynamic effects of mirabegron in nine patients (three men and six women, age 17–68 years) with low‐compliance bladder including seven patients with neurological diseases (three spinal cord injury, four myelomeningocele, and one post‐radical hysterectomy; Kamei et al., 2015). Mirabegron treatment significantly increased first desire to void and cystometric capacity with an average increment of 80 ml (P = 0.027) and 123 ml (P = 0.005) respectively. Bladder compliance also significantly increased (mean value 8.1 ml·cmH2O−1 before, 18.2 ml·cmH2O−1 after, P = 0.024). In the six patients who had been taking anticholinergic agents at a baseline video‐urodynamic study and then switched to mirabegron, mean cystometric capacity and bladder compliance were also increased significantly from 208.3 to 346.8 ml (P = 0.015) and from 7.2 to 17.5 ml·cmH2O−1 (P = 0.047) respectively. Vesicoureteral reflex grade was improved in three of the four patients who had shown vesicoureteral reflex on cystography before treatment. Further prospective studies are needed to confirm the effect of β3‐adrenoceptor agonists on poor compliant detrusor.
6.7. Does desensitization of β3‐adrenoceptors exist in the bladder upon prolonged agonist exposure?
β3‐adrenoceptor agonists can effectively reduce OAB symptoms (Chapple et al., 2014; Ohlstein et al., 2012; Yoshida, Takeda, et al., 2018). As they are not curative, it is assumed that prolonged, possibly life‐long treatment is required for sustained symptom control. Data with β2‐adrenoceptors show that prolonged treatment can cause desensitization, for instance, in the treatment of preterm labour (Engelhardt et al., 1997; Frambach et al., 2005; Michel, Pingsmann, Nohlen, Siekmann, & Brodde, 1989) and this can become treatment‐limiting in patients (Canadian Preterm Labor Investigators, 1992) and animal models thereof (Lye, Dayes, Freitag, Brooks, & Casper, 1992). Whether this also applies to the use of β3‐adrenoceptor agonists in the treatment of OAB remains unclear because the earliest time points of symptom assessment after initiation of treatment have been 2–4 weeks in clinical studies (Chapple et al., 2014; Ohlstein et al., 2012; Yoshida, Takeda, et al., 2018), that is, a time point where desensitization may already have happened.
Many investigators have studied agonist‐induced desensitization of β3‐adrenoceptors in a wide range of animal tissues and of the human subtypes upon endogenous expression and transfection in various cell lines, as reviewed in detail elsewhere in this issue (Okeke, Angers, Bouvier, & Michael, 2019). While there is consensus that β3‐adrenoceptors are less sensitive to agonist‐induced desensitization as compared to β1‐ and β2‐adrenoceptors, whether they exhibit agonist‐induced desensitization and which mechanisms are involved appears to be highly cell type‐dependent. This necessitates studying β3‐adrenoceptor desensitization directly in the urinary bladder.
We are aware of only one study on the urinary bladder (Michel, 2014) in which, in contrast to the human bladder, relaxation is a mixed β2/β3 response (Michel & Vrydag, 2006). Following pretreatment of isolated rat bladder strips for 6 hr with the reference agonist isoprenaline, the β2‐selective fenoterol or the β3‐selective CL316,243 or mirabegron and subsequent washout, concentration–response curves were generated for relaxation by freshly added agonists. Pretreatment with isoprenaline or fenoterol markedly reduced relaxation to freshly added agonist, demonstrating desensitization of the β2‐component by agonists stimulating that subtype. In contrast, pretreatment with CL316,243 or mirabegron caused a much smaller reduction of isoprenaline‐ or fenoterol‐induced relaxation, which did not reach statistical significance. Pretreatment with isoprenaline reduced the response to CL316,243 but not to mirabegron; similarly, pretreatment with CL316,243 reduced relaxation to freshly added agonist (although not reaching statistical significance), whereas pretreatment with mirabegron did not (Table 2). Pretreatment with all four agonists attenuated contractile responses to the muscarinic agonist carbachol or to KCl.
Table 2.
Effects of pretreatment on relaxation responses of isolated rat bladder to β‐adrenocpetor (AR) agonists
| Relaxing agonist | Isoprenaline | Fenoterol | CL316,243 | Mirabegron | ||||
|---|---|---|---|---|---|---|---|---|
| Pretreatment | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax |
| Vehicle | 7.34 ± 0.06 | 49 ± 5 | 6.99 ± 0.23 | 42 ± 3 | 8.45 ± 0.24 | 30 ± 5 | 5.55 ± 0.17 | 45 ± 4 |
| Isoprenaline | 7.01 ± 0.09 | 26 ± 4* | 8.39 ± 0.33* | 19 ± 2* | 8.65 ± 0.35 | 12 ± 3* | 5.52 ± 0.49 | 40 ± 4 |
| Fenoterol | 7.46 ± 0.16 | 24 ± 5* | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| CL316,243 | 7.36 ± 0.02 | 38 ± 3 | 6.63 ± 0.17 | 33 ± 4 | 8.82 ± 0.25 | 20 ± 2 | n.d. | n.d. |
| Mirabegron | 7.08 ± 0.13 | 35 ± 4 | 6.63 ± 0.10 | 34 ± 3 | n.d. | n.d. | 4.84 ± 0.13 | 45 ± 4 |
Note: β‐adrenoceptor agonist potency (pEC50) is given in –log molar units and maximum relaxation (Emax) in %. Data are means ± SEM of 3–8 experiments.
P < 0.05 versus vehicle pretreatment in one‐way ANOVA followed by Dunnett's multiple comparison tests; n.d.: not determined. Reproduced with permission from Michel (2014).
These data confirm in rat bladder that β3‐adrenoceptors are less sensitive to desensitization than other β‐adrenoceptor subtypes. They raise three new questions, which remain to be answered: Is the numerical reduction of relaxation not reaching statistical significance indicating a lack of desensitization or rather an underpowered study? Do β3‐adrenoceptor agonists differ in their ability to cause desensitization of bladder relaxation? Can these findings with rat bladder be extrapolated to the human bladder?
6.8. What is the cause of (rarely observed) cardiovascular effects of mirabegron?
β3‐adrenoceptor agonists as a class appear to be well‐tolerated and have little effect on the cardiovascular system (Rosa et al., 2018). While this general conclusion is largely based on studies with mirabegron, more limited data with solabegron (Ohlstein et al., 2012) and vibegron (Yoshida, Takeda, et al., 2018) confirm it. The molecular basis for the good tolerability appears to be the rather restricted expression of β3‐adrenoceptors in the human body, with their largely lack of expression in the heart (Michel & Gravas, 2016). Nonetheless, some cases of severe hypertension and associated cerebrovascular and cardiac effects have been observed and have led to corresponding warnings in the prescribing information (Medicines and Healthcare Product Regulatory Agency, 2015). While convincing evidence for functional β3‐adrenoceptors in the heart of experimental animals such as rats has been presented (Arioglu‐Inan, Ozakca, Kayki‐Mutlu, Sepici‐Dincel, & Altan, 2013), the data available do not support their presence in the human heart (Michel, Harding, & Bond, 2011). Correspondingly, positive inotropic effects of mirabegron in isolated human atrium have been shown to be resistant to inhibition by β3‐adrenoceptor antagonists (Mo, Michel, Lee, Kaumann, & Molenaar, 2017). Similarly, increases in blood pressure and heart rate induced by supra‐therapeutic doses of mirabegron in healthy volunteers were blocked by propranolol or bisoprolol (van Gelderen et al., 2017). This raises the question what mediates the observed rare but severe cases of hypertension in patients receiving mirabegron treatment.
One possibility would be direct effects of mirabegron on β1‐ or β2‐adrenoceptors. Of note, spare receptors for β‐adrenoceptor‐mediated positive inotropic effects have been reported in the human heart (Brown et al., 1992), indicating that even occupation of a few β1‐ or β2‐adrenoceptors by an agonist could increase cardiac contractility. However, we consider this unlikely for mirabegron because it not only is selective for β3‐adrenoceptors based on affinity but also has a very low intrinsic efficacy at the human β1‐ and β2‐adrenoceptors (Igawa & Michel, 2013). It has been reported that mirabegron has α1‐adrenoceptor antagonist effects in concentrations comparable to its affinity measured at the β3‐adrenoceptors (Alexandre et al., 2016; U.S. Food and Drug Administration, 2012). However, it is questionable whether such concentrations can be reached therapeutically; even if they were, inhibition of α1‐adrenoceptors, if anything, should lower and not increase blood pressure (Michel, 2016).
A recent study has reported that high concentrations of mirabegron can have positive inotropic effects in isolated human right atrium (Mo et al., 2017). These were not affected by the β3‐adrenoceptor antagonist L 748,337 but abolished by the β1‐adrenoceptor antagonist CGP 20,712, indicating the involvement of β1‐adrenoceptors. Given the poor intrinsic efficacy of mirabegron at β1‐adrenoceptors (Igawa & Michel, 2013), the authors investigated other possible underlying mechanisms. Because the neuronal uptake blockers desipramine and phenoxybenzamine also inhibited positive inotropic effects of mirabegron and because mirabegron belongs to the chemical class of phenylethanolamines, many of which are indirect sympathomimetics, they proposed that high concentrations of mirabegron may promote noradrenaline release which in turn may stimulate cardiac β1‐adrenoceptors (Mo et al., 2017). As it remains unclear whether these high concentrations are reached upon administration of therapeutic doses and because no data on human ventricle have been reported, this proposal remains to be proven. However, it remains the only plausible interpretation of the clinically‐observed rare occurrence of severe hypertension. Irrespective of such mechanistic considerations, it is interesting that a pilot study in a small number of patients with congestive heart failure has shown that a 6‐month treatment with mirabegron has a beneficial effect as compared to placebo (Bundgaard et al., 2017).
7. CONCLUSION
The β3‐adrenoceptor agonist mirabegron has become a widely used drug for the treatment of OAB, and other β3‐adrenoceptor agonists such as solabegron and vibegron have shown similar effects in clinical studies. In contrast to the original smooth muscle‐centric view that β3‐adrenoceptors should improve OAB symptoms by directly acting on the detrusor muscle, more recent data suggest that several other cell types may be involved. However, the relative role of the various cellular targets of β3‐adrenoceptor agonists related to bladder function remains to be elucidated.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
CONFLICT OF INTEREST
Y.I. has received consultancy honoraria and/or research support from Astellas, Kissei, and Kyorin. M.C.M. has received consultancy honoraria and/or research support from Apogepha, Astellas, Boehringer Ingelheim, Ferring, Sanofi, and Velicept; he also is a shareholder of Velicept. N.A. reports no conflict of interest.
Igawa Y, Aizawa N, Michel MC. β3‐Adrenoceptors in the normal and diseased urinary bladder—What are the open questions? Br J Pharmacol. 2019;176:2525–2538. 10.1111/bph.14658
REFERENCES
- Abrams, P. , Cardozo, L. , Fall, M. , Griffiths, D. , Rosier, P. , Ulmsten, U. , … Standardisation Sub‐committee of the International Continence Society (2002). The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub‐committee of the International Continence Society. Neurourology and Urodynamics, 21, 167–178. 10.1002/nau.10052 [DOI] [PubMed] [Google Scholar]
- Abrams, P. , Kelleher, C. , Staskin, D. , Kay, R. , Martan, A. , Mincik, I. , … van Maanen, R. (2017). Combination treatment with mirabegron and solifenacin in patients with overactive bladder: Exploratory responder analyses of efficacy and evaluation of patient‐reported outcomes from a randomized, double‐blind, factorial, dose‐ranging, Phase II study (SYMPHONY). World Journal of Urology, 35, 827–838. 10.1007/s00345-016-1908-1 [DOI] [PubMed] [Google Scholar]
- Aizawa, N. , Homma, Y. , & Igawa, Y. (2012). Effects of mirabegron, a novel β3‐adrenoceptor agonist, on primary bladder afferent activity and bladder microcontractions in rats compared with the effects of oxybutynin. European Urology, 62, 1165–1173. 10.1016/j.eururo.2012.08.056 [DOI] [PubMed] [Google Scholar]
- Aizawa, N. , Homma, Y. , & Igawa, Y. (2015). Effects of L‐arginine, mirabegron, and oxybutynin on the primary bladder afferent nerve activities synchronized with reflexic, rhythmic bladder contractions in the rat. Neurourology and Urodynamics, 34, 368–374. 10.1002/nau.22571 [DOI] [PubMed] [Google Scholar]
- Aizawa, N. , Ichihara, K. , Fukuhara, H. , Fujimura, T. , Andersson, K. E. , Homma, Y. , & Igawa, Y. (2017). Characteristics of the mechanosensitive bladder afferent activities in relation with microcontractions in male rats with bladder outlet obstruction. Scientific Reports, 7, 7646 10.1038/s41598-017-07898-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa, N. , Igawa, Y. , Nishizawa, O. , & Wyndaele, J. J. (2010). Effects of CL316,243, a β 3‐adrenoceptor agonist, and intravesical prostaglandin E2 on the primary bladder afferent activity of the rat. Neurourology and Urodynamics, 29, 771–776. 10.1002/nau.20826 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174(Suppl 1), S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre, E. C. , Kiguti, L. R. , Calmasini, F. B. , Silva, F. H. , da Silva, K. P. , Ferreira, R. , … Antunes, E. (2016). Mirabegron relaxes urethral smooth muscle by a dual mechanism involving β3‐adrenoceptor activation and α1‐adrenoceptor blockade. British Journal of Pharmacology, 173, 415–428. 10.1111/bph.13367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, K. E. (1986). Clinical relevance of some findings in neuro‐anatomy and neurophysiology of the lower urinary tract. Clinical Science (London, England: 1979), 70(Suppl 14), 21s–32s. [DOI] [PubMed] [Google Scholar]
- Apodaca, G. (2004). The uroepithelium: Not just a passive barrier. Traffic, 5, 117–128. 10.1046/j.1600-0854.2003.00156.x [DOI] [PubMed] [Google Scholar]
- Arch, J. R. S. , Ainsworth, A. T. , Cawthorne, M. A. , Piercy, V. , Sennitt, M. V. , Thody, V. E. , … Wilson, S. (1984). Atypical β‐adrenoceptor on brown adipocytes as target for anti‐obesity drugs. Nature, 309, 163–165. 10.1038/309163a0 [DOI] [PubMed] [Google Scholar]
- Arioglu‐Inan, E. , Ozakca, I. , Kayki‐Mutlu, G. , Sepici‐Dincel, A. , & Altan, V. M. (2013). The role of insulin‐thyroid hormone interaction on β‐adrenoceptor‐mediated cardiac responses. European Journal of Pharmacology, 718, 533–543. 10.1016/j.ejphar.2013.06.021 [DOI] [PubMed] [Google Scholar]
- Barendrecht, M. M. , Frazier, E. P. , Vrydag, W. , Alewijnse, A. E. , Peters, S. L. , & Michel, M. C. (2009). The effect of bladder outlet obstruction on α1‐ and β‐adrenoceptor expression and function. Neurourology and Urodynamics, 28, 349–355. 10.1002/nau.20642 [DOI] [PubMed] [Google Scholar]
- Bayrak, S. , Balkanci, Z. D. , Pehlivanoglu, B. , Karabulut, I. , Karaismailoglu, S. , & Erdem, A. (2015). Does hypercholesterolemia affect the relaxation of the detrusor smooth muscle in rats? In vitro and in vivo studies. Naunyn‐Schmiedeberg's Archives of Pharmacology, 388, 761–771. 10.1007/s00210-014-1060-7 [DOI] [PubMed] [Google Scholar]
- Beauval, J. B. , Guilloteau, V. , Cappellini, M. , Westfall, T. D. , Rischmann, P. , Palea, S. , … Lluel, P. (2015). Comparison of the effects of β3‐adrenoceptor agonism on urinary bladder function in conscious, anesthetized, and spinal cord injured rats. Neurourology and Urodynamics, 34, 578–585. 10.1002/nau.22629 [DOI] [PubMed] [Google Scholar]
- Biallosterski, B. T. , van Koeveringe, G. A. , van Kerrebroeck, P. E. , Gillespie, J. I. , & de Wachter, S. G. (2011). Nonvoiding activity of the guinea pig bladder. The Journal of Urology, 186, 721–727. 10.1016/j.juro.2011.03.123 [DOI] [PubMed] [Google Scholar]
- Brown, L. , Deighton, N. M. , Bals, S. , Sohlmann, W. , Zerkowski, H. R. , Michel, M. C. , & Brodde, O. E. (1992). Spare receptors for β‐adrenoceptor‐mediated positive inotropic effects of catecholamines in the human heart. Journal of Cardiovascular Pharmacology, 19, 222–232. 10.1097/00005344-199202000-00011 [DOI] [PubMed] [Google Scholar]
- Bundgaard, H. , Axelsson, A. , Hartvig Thomsen, J. , Sorgaard, M. , Kofoed, K. F. , Hasselbalch, R. , … Rasmussen, H. H. (2017). The first‐in‐man randomized trial of a β3 adrenoceptor agonist in chronic heart failure: The BEAT‐HF trial. European Journal of Heart Failure, 19, 566–575. 10.1002/ejhf.714 [DOI] [PubMed] [Google Scholar]
- Calmasini, F. B. , Candido, T. Z. , Alexandre, E. C. , D'Ancona, C. A. , Silva, D. , de Oliveira, M. A. , … Mónica, F. Z. (2015). The β‐3 adrenoceptor agonist, mirabegron relaxes isolated prostate from human and rabbit: New therapeutic indication? Prostate, 75(4), 440–447. 10.1002/pros.22930 [DOI] [PubMed] [Google Scholar]
- Canadian Preterm Labor Investigators G (1992). Treatment of preterm labor with the β‐adrenergic agonist ritodrine. The New England Journal of Medicine, 327, 308–312. [DOI] [PubMed] [Google Scholar]
- Cernecka, H. , Ochodnicky, P. , Lamers, W. H. , & Michel, M. C. (2012). Specificity evaluation of antibodies against human β3‐adrenoceptors. Naunyn‐Schmiedeberg's Archives of Pharmacology, 385, 875–882. 10.1007/s00210-012-0767-6 [DOI] [PubMed] [Google Scholar]
- Cernecka, H. , Pradidarcheep, W. , Lamers, W. H. , Schmidt, M. , & Michel, M. C. (2014). Rat β(3)‐adrenoceptor protein expression: Antibody validation and distribution in rat gastrointestinal and urogenital tissues. Naunyn‐Schmiedeberg's Archives of Pharmacology, 387, 1117–1127. 10.1007/s00210-014-1039-4 [DOI] [PubMed] [Google Scholar]
- Chamberlain, P. D. , Jennings, K. H. , Paul, F. , Cordell, J. , Berry, A. , Holmes, S. D. , … Murphy, G. J. (1999). The tissue distribution of the human β3‐adrenoceptor studied using a monoclonal antibody: Direct evidence of the β3‐adrenoceptor in human adipose tissue, atrium and skeletal muscle. International Journal of Obesity and Related Metabolic Disorders, 23, 1057–1065. 10.1038/sj.ijo.0801039 [DOI] [PubMed] [Google Scholar]
- Chapple, C. R. , Cardozo, L. , Nitti, V. W. , Siddiqui, E. , & Michel, M. C. (2014). Mirabegron in overactive bladder: A review of efficacy, safety, and tolerability. Neurourology and Urodynamics, 33, 17–30. 10.1002/nau.22505 [DOI] [PubMed] [Google Scholar]
- Coelho, A. , Antunes‐Lopes, T. , Gillespie, J. , & Cruz, F. (2017). β‐3 adrenergic receptor is expressed in acetylcholine‐containing nerve fibers of the human urinary bladder: An immunohistochemical study. Neurourology and Urodynamics, 36, 1972–1980. 10.1002/nau.23224 [DOI] [PubMed] [Google Scholar]
- D' Agostino, G. , Maria Condino, A. , & Calvi, P. (2015). Involvement of β3‐adrenoceptors in the inhibitory control of cholinergic activity in human bladder: Direct evidence by [(3)H]‐acetylcholine release experiments in the isolated detrusor. European Journal of Pharmacology, 758, 115–122. 10.1016/j.ejphar.2015.03.074 [DOI] [PubMed] [Google Scholar]
- Dale, P. R. , Cernecka, H. , Schmidt, M. , Dowling, M. R. , Charlton, S. J. , Pieper, M. P. , & Michel, M. C. (2014). The pharmacological rationale for combining muscarinic receptor antagonists and β‐adrenoceptor agonists in the treatment of airway and bladder disease. Current Opinion in Pharmacology, 16, 31–42. 10.1016/j.coph.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat, W. C. (2004). The urothelium in overactive bladder: Passive bystander or active participant? Urology, 64, 7–11. 10.1016/j.urology.2004.08.063 [DOI] [PubMed] [Google Scholar]
- De Matteis, R. , Arch, J. R. , Petroni, M. L. , Ferrari, D. , Cinti, S. , & Stock, M. J. (2002). Immunohistochemical identification of the β(3)‐adrenoceptor in intact human adipocytes and ventricular myocardium: Effect of obesity and treatment with ephedrine and caffeine. International Journal of Obesity and Related Metabolic Disorders, 26, 1442–1450. 10.1038/sj.ijo.0802148 [DOI] [PubMed] [Google Scholar]
- Drake, M. J. , Harvey, I. J. , Gillespie, J. I. , & Van Duyl, W. A. (2005). Localized contractions in the normal human bladder and in urinary urgency. BJU International, 95, 1002–1005. 10.1111/j.1464-410X.2005.05455.x [DOI] [PubMed] [Google Scholar]
- Eastham, J. , Stephenson, C. , Korstanje, K. , & Gillespie, J. I. (2015). The expression of β3‐adrenoceptor and muscarinic type 3 receptor immuno‐reactivity in the major pelvic ganglion of the rat. Naunyn‐Schmiedeberg's Archives of Pharmacology, 388, 695–708. 10.1007/s00210-015-1122-5 [DOI] [PubMed] [Google Scholar]
- Edmondson, S. D. , Zhu, C. , Kar, N. F. , Di Salvo, J. , Nagabukuro, H. , Sacre‐Salem, B. , … Weber, A. E. (2016). Discovery of vibegron: A potent and selective β3 adrenergic receptor agonist for the treatment of overactive bladder. Journal of Medicinal Chemistry, 59, 609–623. 10.1021/acs.jmedchem.5b01372 [DOI] [PubMed] [Google Scholar]
- Ehlert, F. J. , Ahn, S. , Pak, K. J. , Park, G. J. , Sangnil, M. S. , Tran, J. A. , & Matsui, M. (2007). Neuronally released acetylcholine acts on the M2 muscarinic receptor to oppose the relaxant effect of isoproterenol on cholinergic contractions in mouse urinary bladder. The Journal of Pharmacology and Experimental Therapeutics, 322, 631–637. 10.1124/jpet.107.121756 [DOI] [PubMed] [Google Scholar]
- Emorine, L. J. , Marullo, S. , Briend‐Sutren, M. M. , Patey, G. , Tate, K. , Delavier‐Klutchko, C. , & Strosberg, A. (1989). Molecular characterization of the human β 3‐adrenergic receptor. Science, 245, 1118–1121. 10.1126/science.2570461 [DOI] [PubMed] [Google Scholar]
- Engelhardt, S. , Zieger, W. , Kassubek, J. , Michel, M. C. , Lohse, M. J. , & Brodde, O. E. (1997). Tocolytic therapy with fenoterol induces selective down‐regulation of β‐adrenergic receptors in human myometrium. The Journal of Clinical Endocrinology and Metabolism, 82, 1235–1242. 10.1210/jcem.82.4.3885 [DOI] [PubMed] [Google Scholar]
- Fowler, C. J. , Griffiths, D. , & de Groat, W. C. (2008). The neural control of micturition. Nature Reviews. Neuroscience, 9, 453–466. 10.1038/nrn2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frambach, T. , Muller, T. , Freund, S. , Engelhardt, S. , Sutterlin, M. , Lohse, M. J. , & Dietl, J. (2005). Self‐limitation of intravenous tocolysis with β2‐adrenergic agonists is mediated through receptor G protein uncoupling. The Journal of Clinical Endocrinology and Metabolism, 90, 2882–2887. 10.1210/jc.2004-1732 [DOI] [PubMed] [Google Scholar]
- Fujimura, T. , Tamura, K. , Tsutsumi, T. , Yamamoto, T. , Nakamura, K. , Koibuchi, Y. , … Yamaguchi, O. (1999). Expression and possible functional role of the β3‐adrenoceptor in human and rat detrusor muscle. The Journal of Urology, 161, 680–685. 10.1016/S0022-5347(01)61994-3 [DOI] [PubMed] [Google Scholar]
- Gabella, G. , & Davis, C. (1998). Distribution of afferent axons in the bladder of rats. Journal of Neurocytology, 27, 141–155. 10.1023/A:1006903507321 [DOI] [PubMed] [Google Scholar]
- Gary, T. , & Robertson, D. (1994). Lessons learned from dopamine b‐hydroxylase deficiency in humans. Physiology, 9, 35–39. 10.1152/physiologyonline.1994.9.1.35 [DOI] [Google Scholar]
- Gillespie, J. I. , Palea, S. , Guilloteau, V. , Guerard, M. , Lluel, P. , & Korstanje, C. (2012). Modulation of non‐voiding activity by the muscarinergic antagonist tolterodine and the β(3)‐adrenoceptor agonist mirabegron in conscious rats with partial outflow obstruction. BJU International, 110, E132–E142. 10.1111/j.1464-410X.2012.11240.x [DOI] [PubMed] [Google Scholar]
- Gosling, J. A. , Dixon, J. S. , & Jen, P. Y. (1999). The distribution of noradrenergic nerves in the human lower urinary tract. A review. European Urology, 36(Suppl 1), 23–30. 10.1159/000052314 [DOI] [PubMed] [Google Scholar]
- Guillaume, J. L. , Petitjean, F. , Haasemann, M. , Bianchi, C. , Eshdat, Y. , & Strosberg, A. D. (1994). Antibodies for the immunochemistry of the human β 3‐adrenergic receptor. European Journal of Biochemistry, 224, 761–770. 10.1111/j.1432-1033.1994.00761.x [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms, H. H. , Zaagsma, J. , & Van der Wal, B. (1974). β‐adrenoceptor studies. III. On the β‐adrenoceptors in rat adipose tissue. European Journal of Pharmacology, 25, 87–91. 10.1016/0014-2999(74)90098-3 [DOI] [PubMed] [Google Scholar]
- Hashitani, H. , Takano, H. , Fujita, K. , Mitsui, R. , & Suzuki, H. (2011). Functional properties of suburothelial microvessels in the rat bladder. The Journal of Urology, 185, 2382–2391. 10.1016/j.juro.2011.02.046 [DOI] [PubMed] [Google Scholar]
- Hatanaka, T. , Ukai, M. , Watanabe, M. , Someya, A. , Ohtake, A. , Suzuki, M. , … Kaku, S. (2013). Effect of mirabegron, a novel β3‐adrenoceptor agonist, on bladder function during storage phase in rats. Naunyn‐Schmiedeberg's Archives of Pharmacology, 386, 71–78. 10.1007/s00210-012-0814-3 [DOI] [PubMed] [Google Scholar]
- Heppner, T. J. , Layne, J. J. , Pearson, J. M. , Sarkissian, H. , & Nelson, M. T. (2011). Unique properties of muscularis mucosae smooth muscle in guinea pig urinary bladder. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 301, R351–R362. 10.1152/ajpregu.00656.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, A. , McCafferty, G. P. , Riedel, E. , Aiyar, N. , Pullen, M. , Evans, C. , … Hieble, J. P. (2007). GW427353 (solabegron), a novel, selective β3‐adrenergic receptor agonist, evokes bladder relaxation and increases micturition reflex threshold in the dog. The Journal of Pharmacology and Experimental Therapeutics, 323, 202–209. 10.1124/jpet.107.125757 [DOI] [PubMed] [Google Scholar]
- Igawa, Y. , & Michel, M. C. (2013). Pharmacological profile of β3‐adrenoceptor agonists in clinical development for the treatment of overactive bladder syndrome. Naunyn‐Schmiedeberg's Archives of Pharmacology, 386, 177–183. 10.1007/s00210-012-0824-1 [DOI] [PubMed] [Google Scholar]
- Igawa, Y. , Yamazaki, Y. , Takeda, H. , Akahane, M. , Ajisawa, Y. , Yoneyama, T. , & Nishizawa, O. (1998). Possible β 3‐adrenoceptor‐mediated relaxation of the human detrusor. Acta Physiologica Scandinavica, 164, 117–118. 10.1046/j.1365-201X.1998.00406.x [DOI] [PubMed] [Google Scholar]
- Igawa, Y. , Yamazaki, Y. , Takeda, H. , Hayakawa, K. , Akahane, M. , Ajisawa, Y. , … Andersson, K. E. (1999). Functional and molecular biological evidence for a possible β3‐adrenoceptor in the human detrusor muscle. British Journal of Pharmacology, 126, 819–825. 10.1038/sj.bjp.0702358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jositsch, G. , Papadakis, T. , Haberberger, R. V. , Wolff, M. , Wess, J. , & Kummer, W. (2009). Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene‐deficient mice. Naunyn‐Schmiedeberg's Archives of Pharmacology, 379, 389–395. 10.1007/s00210-008-0365-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidoh, K. , Igawa, Y. , Takeda, H. , Yamazaki, Y. , Akahane, S. , Miyata, H. , … Andersson, K. E. (2002). Effects of selective β2 and β3‐adrenoceptor agonists on detrusor hyperreflexia in conscious cerebral infarcted rats. The Journal of Urology, 168, 1247–1252. 10.1016/S0022-5347(05)64634-4 [DOI] [PubMed] [Google Scholar]
- Kamei, J. , Furuta, A. , Akiyama, Y. , Niimi, A. , Ichihara, K. , Fujimura, T. , … Igawa, Y. (2015). Video‐urodynamic effects of mirabegron, a β3‐adrenoceptor agonist, in patients with low‐compliance bladder. International Journal of Urology, 22, 956–961. 10.1111/iju.12867 [DOI] [PubMed] [Google Scholar]
- Kanai, A. , Zabbarova, I. , Oefelein, M. , Radziszewski, P. , Ikeda, Y. , & Andersson, K. E. (2012). Mechanisms of action of botulinum neurotoxins, β3‐adrenergic receptor agonists, and PDE5 inhibitors in modulating detrusor function in overactive bladders: ICI‐RS 2011. Neurourology and Urodynamics, 31, 300–308. 10.1002/nau.21246 [DOI] [PubMed] [Google Scholar]
- Klausner, A. P. , Rourke, K. F. , Miner, A. S. , & Ratz, P. H. (2009). Potentiation of carbachol‐induced detrusor smooth muscle contractions by β‐adrenoceptor activation. European Journal of Pharmacology, 606, 191–198. 10.1016/j.ejphar.2009.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korstanje, C. , Krauwinkel, W. , & van Doesum‐Wolters, F. L. (2011). Tamsulosin shows a higher unbound drug fraction in human prostate than in plasma: A basis for uroselectivity? British Journal of Clinical Pharmacology, 72, 218–225. 10.1111/j.1365-2125.2010.03870.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauwinkel, W. , van Dijk, J. , Schaddelee, M. , Eltink, C. , Meijer, J. , Strabach, G. , … van Gelderen, M. (2012). Pharmacokinetic properties of mirabegron, a β3‐adrenoceptor agonist: Results from two phase I, randomized, multiple‐dose studies in healthy young and elderly men and women. Clinical Therapeutics, 34, 2144–2160. 10.1016/j.clinthera.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Kushida, N. , & Fry, C. H. (2016). On the origin of spontaneous activity in the bladder. BJU International, 117, 982–992. 10.1111/bju.13240 [DOI] [PubMed] [Google Scholar]
- Limberg, B. J. , Andersson, K. E. , Aura Kullmann, F. , Burmer, G. , de Groat, W. C. , & Rosenbaum, J. S. (2010). β‐Adrenergic receptor subtype expression in myocyte and non‐myocyte cells in human female bladder. Cell and Tissue Research, 342, 295–306. 10.1007/s00441-010-1053-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye, S. J. , Dayes, B. A. , Freitag, C. L. , Brooks, J. , & Casper, R. F. (1992). Failure of ritodrine to prevent preterm labor in the sheep. American Journal of Obstetrics and Gynecology, 167, 1399–1408. 10.1016/S0002-9378(11)91725-6 [DOI] [PubMed] [Google Scholar]
- Maruyama, I. , Goi, Y. , Tatemichi, S. , Maruyama, K. , Hoyano, Y. , Yamazaki, Y. , & Kusama, H. (2012). Bladder selectivity of the novel β(3)‐agonist ritobegron (KUC‐7483) explored by in vitro and in vivo studies in the rat. Naunyn‐Schmiedeberg's Archives of Pharmacology, 385, 845–852. 10.1007/s00210-012-0755-x [DOI] [PubMed] [Google Scholar]
- Matsumoto, R. , Otsuka, A. , Suzuki, T. , Shinbo, H. , Mizuno, T. , Kurita, Y. , … Ozono, S. (2013). Expression and functional role of β3‐adrenoceptors in the human ureter. International Journal of Urology, 20, 1007–1014. 10.1111/iju.12093 [DOI] [PubMed] [Google Scholar]
- Medicines and Healthcare Products Regulatory Ageny . (2015). Mirabegron (Betmiga): risk of severe hypertension and associated cerebrovascular and cardiac effects. Available from https://www.gov.uk/drug-safety-update/mirabegron-betmiga-risk-of-severe-hypertension-and-associated-cerebrovascular-and-cardiac-events. [Accessed July 8 2016].
- Michel, M. C. (2014). Do β‐adrenoceptor agonists induce homologous or heterologous desensitization in rat urinary bladder? Naunyn‐Schmiedeberg's Archives of Pharmacology, 387, 215–224. 10.1007/s00210-013-0936-2 [DOI] [PubMed] [Google Scholar]
- Michel, M. C. (2016). How β3‐adrenoceptor‐selective is mirabegron? British Journal of Pharmacology, 173, 429–430. 10.1111/bph.13379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, M. C. , & Chapple, C. R. (2009). Basic mechanisms of urgency: Preclinical and clinical evidence. European Urology, 56, 298–307. 10.1016/j.eururo.2009.05.028 [DOI] [PubMed] [Google Scholar]
- Michel, M. C. , & Gravas, S. (2016). Safety and tolerability of β3‐adrenoceptor agonists in the treatment of overactive bladder syndrome—Insight from transcriptosome and experimental studies. Expert Opinion on Drug Safety, 15, 647–657. 10.1517/14740338.2016.1160055 [DOI] [PubMed] [Google Scholar]
- Michel, M. C. , Harding, S. E. , & Bond, R. A. (2011). Are there functional β(3)‐adrenoceptors in the human heart? British Journal of Pharmacology, 162, 817–822. 10.1111/j.1476-5381.2010.01005.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, M. C. , & Korstanje, C. (2016). β3‐Adrenoceptor agonists for overactive bladder syndrome: Role of translational pharmacology in a repositioning clinical drug development project. Pharmacology & Therapeutics, 159, 66–82. 10.1016/j.pharmthera.2016.01.007 [DOI] [PubMed] [Google Scholar]
- Michel, M. C. , Ochodnicky, P. , Homma, Y. , & Igawa, Y. (2011). β‐adrenoceptor agonist effects in experimental models of bladder dysfunction. Pharmacology & Therapeutics, 131, 40–49. 10.1016/j.pharmthera.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Michel, M. C. , Pingsmann, A. , Nohlen, M. , Siekmann, U. , & Brodde, O. E. (1989). Decreased myometrial β‐adrenoceptors in women receiving β 2‐adrenergic tocolytic therapy: Correlation with lymphocyte β‐adrenoceptors. Clinical Pharmacology and Therapeutics, 45, 1–8. 10.1038/clpt.1989.1 [DOI] [PubMed] [Google Scholar]
- Michel, M. C. , & Sand, C. (2009). Effect of pre‐contraction on β‐adrenoceptor‐mediated relaxation of rat urinary bladder. World Journal of Urology, 27, 711–715. 10.1007/s00345-009-0416-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, M. C. , & Vrydag, W. (2006). α1‐, α2‐ and β‐adrenoceptors in the urinary bladder, urethra and prostate. British Journal of Pharmacology, 147(Suppl 2), S88–S119. 10.1038/sj.bjp.0706619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo, W. , Michel, M. C. , Lee, X. W. , Kaumann, A. J. , & Molenaar, P. (2017). The β3‐adrenoceptor agonist mirabegron increases human atrial force through β1‐adrenoceptors: An indirect mechanism? British Journal of Pharmacology, 174, 2706–2715. 10.1111/bph.13897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro, C. , Leeds, C. , & Chess‐Williams, R. (2012). Contractile activity of the bladder urothelium/lamina propria and its regulation by nitric oxide. European Journal of Pharmacology, 674, 445–449. 10.1016/j.ejphar.2011.11.020 [DOI] [PubMed] [Google Scholar]
- Moro, C. , Uchiyama, J. , & Chess‐Williams, R. (2011). Urothelial/lamina propria spontaneous activity and the role of M3 muscarinic receptors in mediating rate responses to stretch and carbachol. Urology, 78, 1442 e1449–1442 e1415. [DOI] [PubMed] [Google Scholar]
- Nahmias, C. , Blin, N. , Elalouf, J. M. , Mattei, M. G. , Strosberg, A. D. , & Emorine, L. J. (1991). Molecular characterization of the mouse β 3‐adrenergic receptor: Relationship with the atypical receptor of adipocytes. The EMBO Journal, 10, 3721–3727. 10.1002/j.1460-2075.1991.tb04940.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nergardh, A. , Boreus, L. O. , & Naglo, A. S. (1977). Characterization of the adrenergic β‐receptor in the urinary bladder of man and cat. Acta Pharmacologica et Toxicologica, 40, 14–21. [DOI] [PubMed] [Google Scholar]
- Nitti, V. W. , Rosenberg, S. , Mitcheson, D. H. , He, W. , Fakhoury, A. , & Martin, N. E. (2013). Urodynamics and safety of the β(3)‐adrenoceptor agonist mirabegron in males with lower urinary tract symptoms and bladder outlet obstruction. The Journal of Urology, 190, 1320–1327. 10.1016/j.juro.2013.05.062 [DOI] [PubMed] [Google Scholar]
- Nomiya, M. , & Yamaguchi, O. (2003). A quantitative analysis of mRNA expression of α 1 and β‐adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. The Journal of Urology, 170, 649–653. 10.1097/01.ju.0000067621.62736.7c [DOI] [PubMed] [Google Scholar]
- Ochodnicky, P. , Humphreys, S. , Eccles, R. , Poljakovic, M. , Wiklund, P. , & Michel, M. C. (2012). Expression profiling of G‐protein‐coupled receptors in human urothelium and related cell lines. BJU International, 110, E293–E300. 10.1111/j.1464-410X.2012.011145.x [DOI] [PubMed] [Google Scholar]
- Ohlstein, E. H. , von Keitz, A. , & Michel, M. C. (2012). A multicenter, double‐blind, randomized, placebo‐controlled trial of the β3‐adrenoceptor agonist solabegron for overactive bladder. European Urology, 62, 834–840. 10.1016/j.eururo.2012.05.053 [DOI] [PubMed] [Google Scholar]
- Okeke, K. , Angers, S. , Bouvier, M. , & Michael, M. C. (2019). Agonist‐induced desensitisation of β3 ‐adrenoceptors: Where, when, and how? British Journal of Pharmacology. 10.1111/bph.14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeke, K. , Gravas, S. , & Michel, M. C. (2017). Do β3‐adrenoceptor agonists cause urinary bladder smooth muscle relaxation by inhibiting acetylcholine release? American Journal of Physiology. Renal Physiology, 313, F859–F861. 10.1152/ajprenal.00215.2017 [DOI] [PubMed] [Google Scholar]
- Otsuka, A. , Kawasaki, H. , Matsumoto, R. , Shinbo, H. , Kurita, Y. , Iwashita, T. , & Ozono, S. (2013). Expression of β‐adrenoceptor subtypes in urothelium, interstitial cells and detrusor of the human urinary bladder. Low Urinary Tract Symptoms, 5, 173–180. 10.1111/luts.12007 [DOI] [PubMed] [Google Scholar]
- Otsuka, A. , Shinbo, H. , Matsumoto, R. , Kurita, Y. , & Ozono, S. (2008). Expression and functional role of β‐adrenoceptors in the human urinary bladder urothelium. Naunyn‐Schmiedeberg's Archives of Pharmacology, 377, 473–481. 10.1007/s00210-008-0274-y [DOI] [PubMed] [Google Scholar]
- Ouslander, J. G. (2004). Management of overactive bladder. The New England Journal of Medicine, 350, 786–799. 10.1056/NEJMra032662 [DOI] [PubMed] [Google Scholar]
- Palea, S. , Rekik, M. , Rouget, C. , Camparo, P. , Botto, H. , Rischmann, P. , … Westfall, T. D. (2012). Fenoterol functionally activates the β₃‐adrenoceptor in human urinary bladder, comparison with rat and mouse: Implications for drug discovery. European Journal of Pharmacology, 690, 202–206. 10.1016/j.ejphar.2012.06.036 [DOI] [PubMed] [Google Scholar]
- Roberts, S. J. , Papaioannou, M. , Evans, B. A. , & Summers, R. J. (1999). Characterization of β‐adrenoceptor mediated smooth muscle relaxation and the detection of mRNA for β1‐, β2‐ and β3‐adrenoceptors in rat ileum. British Journal of Pharmacology, 127, 949–961. 10.1038/sj.bjp.0702605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa, G. M. , Baccino, D. , Valbusa, A. , Scala, C. , Barra, F. , Brunelli, C. , & Ferrero, S. (2018). Cardiovascular effects of antimuscarinic agents and β3‐adrenergic receptor agonist for the treatment of overactive bladder. Expert Opinion on Drug Safety, 17, 487–497. 10.1080/14740338.2018.1453496 [DOI] [PubMed] [Google Scholar]
- Rouget, C. , Rekik, M. , Camparo, P. , Botto, H. , Rischmann, P. , Lluel, P. , … Westfall, T. D. (2014). Modulation of nerve‐evoked contractions by β3‐adrenoceptor agonism in human and rat isolated urinary bladder. Pharmacological Research, 80, 14–20. 10.1016/j.phrs.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Sadananda, P. , Drake, M. J. , Paton, J. F. , & Pickering, A. E. (2013). A functional analysis of the influence of β3‐adrenoceptors on the rat micturition cycle. The Journal of Pharmacology and Experimental Therapeutics, 347, 506–515. 10.1124/jpet.113.207340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada, N. , Nomiya, M. , Hood, B. , Koslov, D. , Zarifpour, M. , & Andersson, K. E. (2013). Protective effect of a β3‐adrenoceptor agonist on bladder function in a rat model of chronic bladder ischemia. European Urology, 64, 664–671. 10.1016/j.eururo.2013.06.043 [DOI] [PubMed] [Google Scholar]
- Sellers, D. , Chess‐Williams, R. , & Michel, M. C. (2018). Modulation of lower urinary tract smooth muscle contraction and relaxation by the urothelium. Naunyn‐Schmiedeberg's Archives of Pharmacology, 391, 675–694. 10.1007/s00210-018-1510-8 [DOI] [PubMed] [Google Scholar]
- Silva, I. , Costa, A. F. , Moreira, S. , Ferreirinha, F. , Magalhaes‐Cardoso, M. T. , Calejo, I. , … Correia‐de‐Sá, P. (2017). Inhibition of cholinergic neurotransmission by β3‐adrenoceptors depends on adenosine release and A1‐receptor activation in human and rat urinary bladders. American Journal of Physiology. Renal Physiology, 313, F388–F403. 10.1152/ajprenal.00392.2016 [DOI] [PubMed] [Google Scholar]
- Suzuki, T. , Otsuka, A. , Matsumoto, R. , Furuse, H. , & Ozono, S. (2016). The expression of β3‐adrenoceptors and their function in the human prostate. The Prostate, 76, 163–171. 10.1002/pros.23108 [DOI] [PubMed] [Google Scholar]
- Svalo, J. , Nordling, J. , Bouchelouche, K. , Andersson, K. E. , Korstanje, C. , & Bouchelouche, P. (2013). The novel β3‐adrenoceptor agonist mirabegron reduces carbachol‐induced contractile activity in detrusor tissue from patients with bladder outflow obstruction with or without detrusor overactivity. European Journal of Pharmacology, 699, 101–105. 10.1016/j.ejphar.2012.11.060 [DOI] [PubMed] [Google Scholar]
- Takasu, T. , Ukai, M. , Sato, S. , Matsui, T. , Nagase, I. , Maruyama, T. , … Yamaguchi, O. (2007). Effect of (R)‐2‐(2‐aminothiazol‐4‐yl)‐4′‐{2‐[(2‐hydroxy‐2‐phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective β3‐adrenoceptor agonist, on bladder function. The Journal of Pharmacology and Experimental Therapeutics, 321, 642–647. 10.1124/jpet.106.115840 [DOI] [PubMed] [Google Scholar]
- Takeda, H. , Yamazaki, Y. , Igawa, Y. , Kaidoh, K. , Akahane, S. , Miyata, H. , … Andersson, K. E. (2002). Effects of β(3)‐adrenoceptor stimulation on prostaglandin E(2)‐induced bladder hyperactivity and on the cardiovascular system in conscious rats. Neurourology and Urodynamics, 21, 558–565. 10.1002/nau.10034 [DOI] [PubMed] [Google Scholar]
- Takeda, M. , Obara, K. , Mizusawa, T. , Tomita, Y. , Arai, K. , Tsutsui, T. , … Nomura, S. (1999). Evidence for β3‐adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: Analysis by molecular biological and pharmacological methods. The Journal of Pharmacology and Experimental Therapeutics, 288, 1367–1373. [PubMed] [Google Scholar]
- Thiagamoorthy, G. , Giarenis, I. , & Cardozo, L. (2015). Early investigational β3 adreno‐receptor agonists for the management of the overactive bladder syndrome. Expert Opinion on Investigational Drugs, 24, 1299–1306. 10.1517/13543784.2015.1076390 [DOI] [PubMed] [Google Scholar]
- Tyagi, P. , Thomas, C. A. , Yoshimura, N. , & Chancellor, M. B. (2009). Investigations into the presence of functional β1, β2 and β3‐adrenoceptors in urothelium and detrusor of human bladder. International Brazilian Journal of Urology, 35, 76–83. 10.1590/S1677-55382009000100012 [DOI] [PubMed] [Google Scholar]
- Uehling, D. E. , Shearer, B. G. , Donaldson, K. H. , Chao, E. Y. , Deaton, D. N. , Adkison, K. K. , … Cowan, C. (2006). Biarylaniline phenethanolamines as potent and selective β3 adrenergic receptor agonists. Journal of Medicinal Chemistry, 49, 2758–2771. 10.1021/jm0509445 [DOI] [PubMed] [Google Scholar]
- Uhlen, M. , Fagerberg, L. , Hallstrom, B. M. , Lindskog, C. , Oksvold, P. , Mardinoglu, A. , … Ponten, F. (2015). Proteomics. Tissue‐based map of the human proteome. Science, 347, 1260419 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration . (2012). Pharmacology/Toxicology NDA/BLA Review and Evaluation (NDA 202–611). Available from http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202611Orig1s000PharmR.pdf. [Accessed May 19 2015].
- van Gelderen, M. , Stolzel, M. , Meijer, J. , Kerbusch, V. , Collins, C. , & Korstanje, C. (2017). An exploratory study in healthy male subjects of the mechanism of mirabegron‐induced cardiovascular effects. Journal of Clinical Pharmacology, 57, 1534–1544. 10.1002/jcph.952 [DOI] [PubMed] [Google Scholar]
- Vera, P. L. , & Nadelhaft, I. (2000). Anatomical evidence for two spinal ‘afferent‐interneuron‐efferent’ reflex pathways involved in micturition in the rat: A ‘pelvic nerve’ reflex pathway and a ‘sacrolumbar intersegmental’ reflex pathway. Brain Research, 883, 107–118. 10.1016/S0006-8993(00)02732-3 [DOI] [PubMed] [Google Scholar]
- Wada, N. , Okazaki, S. , Kobayashi, S. , Hashizume, K. , Kita, M. , Matsumoto, S. , & Kakizaki, H. (2015). Efficacy of combination therapy with mirabegron for anticholinergic‐resistant neurogenic bladder: Videourodynamic evaluation. Hinyokika Kiyo Acta Urologica Japonica, 61, 7–11. [PubMed] [Google Scholar]
- Wada, N. , Shimizu, T. , Takai, S. , Shimizu, N. , Tyagi, P. , Kakizaki, H. , & Yoshimura, N. (2017). Combinational effects of muscarinic receptor inhibition and β3‐adrenoceptor stimulation on neurogenic bladder dysfunction in rats with spinal cord injury. Neurourology and Urodynamics, 36, 1039–1045. 10.1002/nau.23066 [DOI] [PubMed] [Google Scholar]
- Wollner, J. , & Pannek, J. (2016). Initial experience with the treatment of neurogenic detrusor overactivity with a new β‐3 agonist (mirabegron) in patients with spinal cord injury. Spinal Cord, 54, 78–82. 10.1038/sc.2015.195 [DOI] [PubMed] [Google Scholar]
- Woods, M. , Carson, N. , Norton, N. W. , Sheldon, J. H. , & Argentieri, T. M. (2001). Efficacy of the β3‐adrenergic receptor agonist CL‐316243 on experimental bladder hyperreflexia and detrusor instability in the rat. The Journal of Urology, 166, 1142–1147. 10.1016/S0022-5347(05)65936-8 [DOI] [PubMed] [Google Scholar]
- Yoshida, M. , Kakizaki, H. , Takahashi, S. , Nagai, S. , & Kurose, T. (2018). Long‐term safety and efficacy of the novel β3‐adrenoreceptor agonist vibegron in Japanese patients with overactive bladder: A phase III prospective study. International Journal of Urology, 25, 668–675. 10.1111/iju.13596 [DOI] [PubMed] [Google Scholar]
- Yoshida, M. , Takeda, M. , Gotoh, M. , Nagai, S. , & Kurose, T. (2018). Vibegron, a novel potent and selective β3‐adrenoreceptor agonist, for the treatment of patients with overactive bladder: A randomized, double‐blind, placebo‐controlled phase 3 study. European Urology, 73, 783–790. 10.1016/j.eururo.2017.12.022 [DOI] [PubMed] [Google Scholar]


