Figure 1.
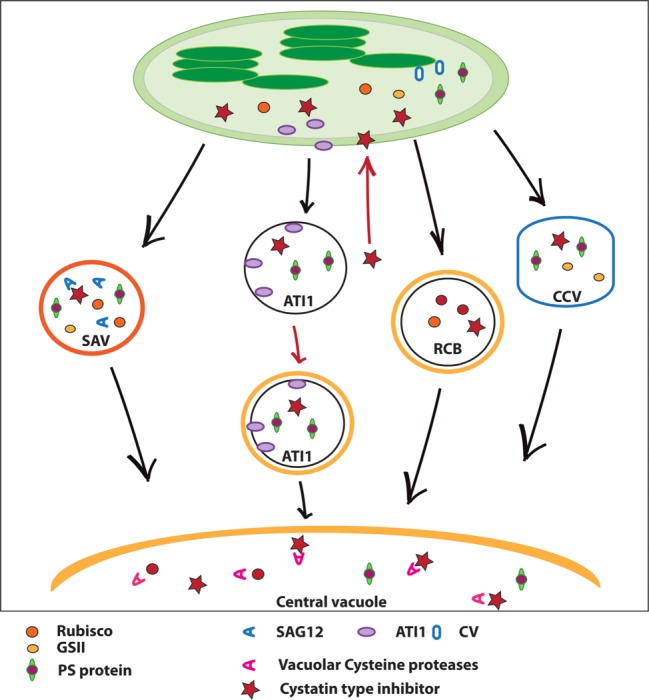
A schematic representation of cellular pathways delivering chloroplast proteins to lytic compartments and a proposed strategy to inhibit plastid protein degradation in crop species. Chloroplast proteins traffic to “senescence-associated vacuoles” (SAVs) and/or to the central vacuole via “ATI1-plastid associated bodies” (ATI1-PS), “Rubisco-containing bodies” (RCBs), or “CV-containing vesicles” (CCVs). Each of these vesicles apparently transports specific proteins (Rubisco for SAVs and RCBs, certain thylakoid proteins for ATI1-PS and CCV), which may be degraded by cysteine proteases either in SAVs or the central vacuole. SAVs might also be internalized into the central vacuole, although this is hypothetical. A proposed strategy to reduce/slow down chloroplast protein degradation during senescence in crop plants is the use of targeted protease inhibitors. A proteinaceous protease inhibitor (e.g., a phytocystatin, or a Kunitz-type inhibitor such as WSCP, represented by a red star in the cytosol) fused to a chloroplast transit peptide is expressed under control of a senescence-induced promoter. The chloroplast-located inhibitor would then be redirected to RCBs, ATI1-PS, CCV, and/or SAVs by the same mechanism which directs chloroplast-targeted fluorescent proteins to these vesicular structures. Once in the central vacuole or in SAVs, the inhibitor binds proteases, thus reducing proteolytic activity and preserving protein levels.
