Figure 2.
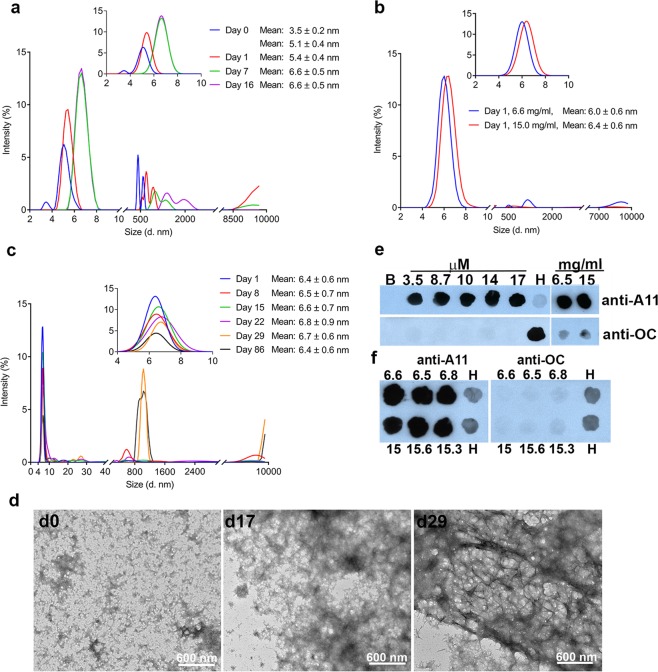
CRES assembles into a stable oligomeric intermediate. (a) CRES preparation #4 from Fig. 1 (purple data set) was followed over 16 days by DLS to examine its aggregation properties in solution. Inset, average hydrodynamic radius for the particles between 4–8 nm was calculated from the fitted data and diameter ± SD is reported. Data are representative of n = 3 different protein preparations (see Supplementary Fig. S5). DLS analysis of (b) mid- (6.6 mg/ml) (blue) and high (15 mg/ml) (red) concentration CRES in 4 mM potassium phosphate buffer, pH 7.4, 24 hours after purification and concentration. Inset, average diameter ± SD of particles in the 4–8 nm population; and (c) high concentration CRES over time (day 1-day 86). Inset, average diameter ± SD of particles in the 4–8 nm population. Data are representative of n = 3 protein preparations (see Supplementary Figs S4 and S5). (d) Negative stain TEM of high concentration CRES at day 0, 17, and 29. (e) Dot blot analysis of samples from different stock concentrations of CRES (3.5–17 μM) in high salt gel filtration buffer and CRES in 4 mM potassium phosphate buffer, pH 7.4 concentrated to 6.5 mg/ml and 15 mg/ml. All were examined within 24 hours after purification. (f) Dot blot analysis of three sets of CRES samples concentrated to mid- (6.5–6.8 mg/ml) and high (15–15.6 mg/ml) concentrations and examined after 45, 60, and 105 days, respectively. For all dot blots 4.4 μg protein was spotted onto nitrocellulose membranes followed by incubation with anti-oligomeric A11 and anti-fibrillar OC antibodies. B, buffer only. H, His-CRES.