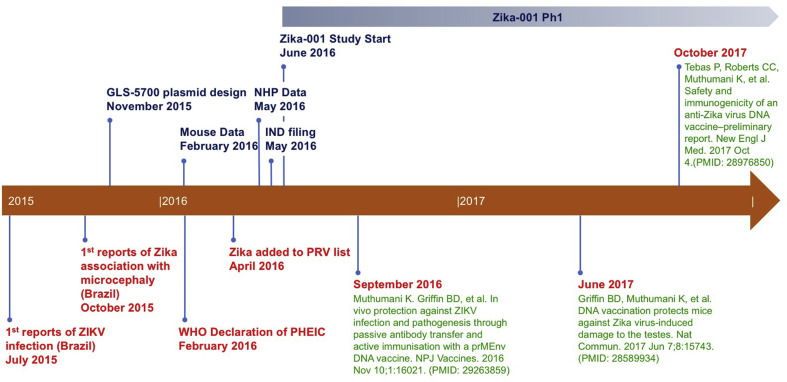
Fig. 2.
Timeline of Zika DNA vaccine development. July of 2015 marked the first reports of Zika infection in Brazil with the first association of Zika with microcephaly reported in October of 2015. Following that report, we started plasmid design and in vitro testing. In February of 2016, about the same time that the WHO declared that Zika was a “Public Health Emergency of International Concern”, we completed the first mouse studies and had begun NHP immunogenicity studies. In May, 2017, the first NHP study was completed and the IND has filed shortly after. The GLS-5700 phase 1 clinical trial started in June 2017, a short 7 months after the initial vaccine design was started (NCT02809443). All the preclinical work has since been published in September of 2016 and June of 2017, and the preliminary report of the clinical trial data was published in October 2017.