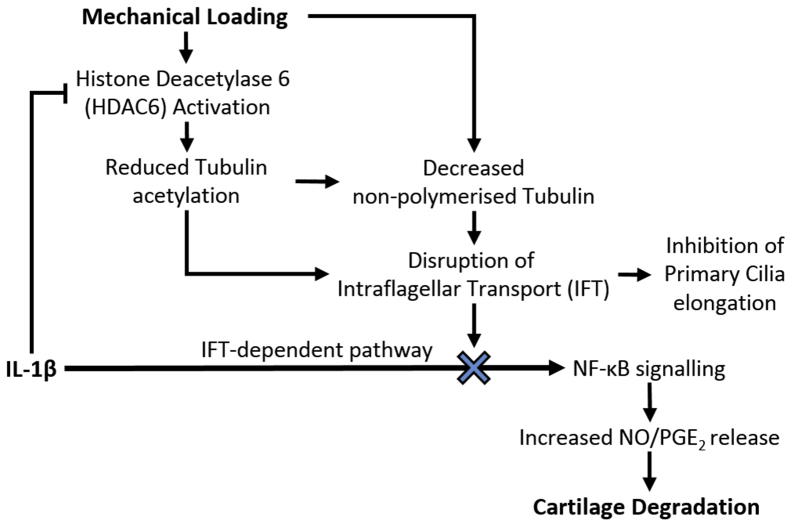
Fig. 7.
Proposed mechanism through which physiological mechanical loading suppresses IL-1β-induced NO/PGE2 production and downstream cartilage degradation. We suggest that the anti-inflammatory effects of mechanical loading are mediated by mechanosensitive HDAC6 activation resulting in disruption of tubulin acetylation and a reduction in the availability of non-polymerized tubulin, which disrupt IFT and associated primary cilia elongation. This mechanical modulation of IFT therefore suppresses IFT-dependent IL-1β signalling events in the form of NO and PGE2 release, thereby reducing cartilage degradation.