Abstract
Background
Rapid increase of products containing titanium leads to the increases in percutaneous and permucosal exposure of populations to the titanium.
Purpose
Evaluate the various material compositions in five commercially available dental implant systems and correlate the obtained material contents with allergic conditions seen with implants.
Material and methods
A total of 25 implant, with 5 samples each in 5 groups of commercially available dental implants (MKIII, Myriad, Nobel Replace, MIS and Alpha Dent) were used in the study. Positive Material Identification (PMI) testing was done to analyse the amount of different metals (percentage by mass) present in the dental implants.
Results
Highest titanium content, 99.47% by mass was found in sample 2 (Myriad) and least, 89.04% by mass in sample 5(Alpha dent). Nickel was found only in sample 5 (Alpha dent) in 0.079% by mass and zinc in sample 4 (MIS) in0.084% by mass, chromium was found in sample 1 (MK III) in 0.263% by mass and in sample 2 in0.273% by mass.
Conclusions
Implant composition should be made mandatory to be disclosed on the implant packet and before implant placement patch test for the allergen present in the particular implant can be done for the patient's health benefit and long term clinical success of dental implants.
Keywords: Dental implant, Metal allergy, Patch test, Positive material identification, Titanium allergy
1. Introduction
In 1965 Prof. Brånemark PI discovered the beneficial effects of titanium in bone healing and later titanium has emerged as the successful material for the rehabilitation of patient with missing tooth.1 Titanium is known to have good biocompatibility, and today titanium is used widely making for dental implants. In most of the long term studies in the literature with a survival rate of more than 95%, implant treatment has emerged as one of the more successful treatment modalities.2
Crestal bone loss (CBL) of implant is considered to occur due to multiple factors which include mechanical overloading, perimplantopathogens and immune reactions. Overloading is considered as the level of force and/or nature of force applied which exceeds the tolerable limit of the prosthesis and biological limit to resist CBL and thus can leads to peri-implantitis. Osteocyte network is regulated by osteoblast-osteoclast axis which contributes to mechanosensory response to loading and leads to apoptosis of osteocyte and targeted bone resorption by osteoclasts.3
Today titanium is used widely for medical applications mainly as titanium dioxide. There is rapid increase of products containing titanium, which leads to the increases in percutaneous and permucosal exposure of populations to the titanium. When compared to other metals such as nickel, palladium, chromium, mercury etc. Prevalence of allergy-positive reactions against titanium alloy is very less. When compared to materials like Cobalt-chromium and stainless steel, titanium alloys have better corrosion properties.4
Metal ions and particles may induce immune responses leading to osteolysis and failure of implants.5 Ionic and particulate titanium particles may influence the immune system as they are considered prone to bind DNA and RNA and induce molecular damage.6 The ions leached in the surrounding induce osteolytic cytokines into tissues leading to loosening of implants and in certain cases may cause severe hypersensitivity or allergic reactions.7 Immune reaction to any foreign-body occurs when the immune response is prolonged or too vigorous or when it disrupts its function. Whenever the immune response to titanium dental implants is associated with other factors or health conditions that increases the immune response, in such case the balance between osteoblast and osteoclast activity during healing phase shift from a net bone apposition to a net bone resorption. This results in failure of osseointegration and CBL by shifting the defense/repair balance towards chronic inflammation and destruction of the tissues.3,8,9
Studies have reported allergic reactions to titanium in cases rehabilitated with titanium-based materials.10 An allergy may be defined as acute immunological responses that occur when coming into contact with a known antigen. Allergy can either be an immediate humoral response due to antibody and antigen complexes seen in type I, II, and III reactions, or delayed due to cell-mediated response seen in type IV reactions. In cases of implant mediated allergic reaction, type IV delayed hypersensitivity is seen typically.11,12
Dental implants failures are have very well documented in the literature. One of the early indication of crestal bone loss in dental implant may be related to multiple factors.13Titanium and titanium alloys are the gold standard for endo-osseus dental implants production, thanks to their biocompatibility, resistance to corrosion and mechanical properties. The characteristics of the titanium implant surface seem to be particularly relevant in the early phase of osseointegration.14Ti alloys have shown integration with bone and soft tissue environments. However, there is concern that Ti alloys contain significant amounts of alloying elements that may affect osseointegration especially due to corrosion products containing aluminium and vanadium.11
Hence, the present study was undertaken to evaluate the various material compositions in five commercially available dental implant systems using Positive Material Identification (PMI) testing. The second objective was to correlate the obtained material contents with allergic conditions seen with implants as presented in literatures.
2. Materials and Methods
The study consists of a total of 25 implant based on statistical sample strength with 5 samples each in 5 groups of commercially available dental implants: Group 1: Branemark system Mk III (Nobel Biocare, Zurich), Group 2: Myriad system (Equinox Medical Technologies B·V,Netherlands), Group 3: Nobel Replace (Nobel Biocare, Zurich), Group 4: MIS Seven (MIS implant system, Austria) and Group 5: Alpha Dent Classic (Alpha dent implant system, Germany.) were used in the study (Fig. 1A,B,1C,1D,1E). The ethical clearance for the study was obtained from the ethical committee of the institute. ThePMI testing for determining the alloy composition of implants was conducted in SIGMA test and Research Centre Bengaluru, Karnataka, India.
Fig. 1.
A.MK III implant, B. Myriad implant, C. Nobel replaces implant, D. MIS implant, E. Alpha dent classic implant.
A single blind trial was done and implants were randomly allocated and labeled as sample 1 (MKIII implant), sample 2 (Myriad implant), sample 3(Nobel Replace implant), Sample 4 (MIS Seven implant) and sample 5 (Alpha dent implant). Implants were packed in separate plastic bag to maintain a dry atmosphere. PMI machine (Thermo-scientific Niton XL3t GOLDD + XRF analyzer) (Fig. 2) was placed against each implant for 30 seconds and elemental analysis of each implant was done and recorded. PMI machine works on principle of X ray fluorescence (XRF) and helps in determining the alloy composition of materials in percentage by mass.
Fig. 2.

PMI testing equipment to evaluate the elemental analysis of dental implants.
3. Results
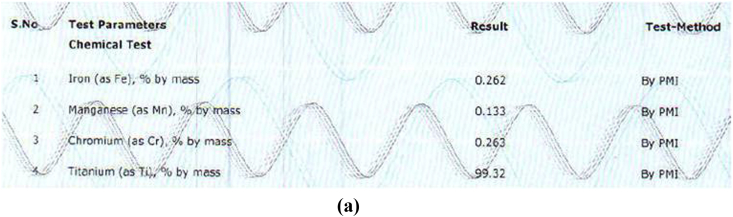
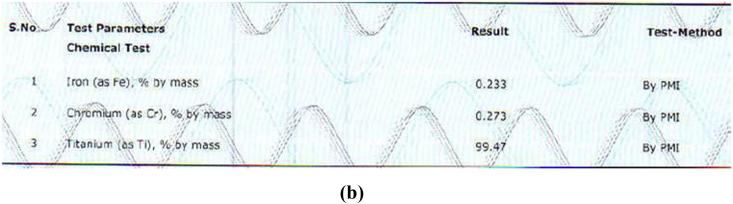
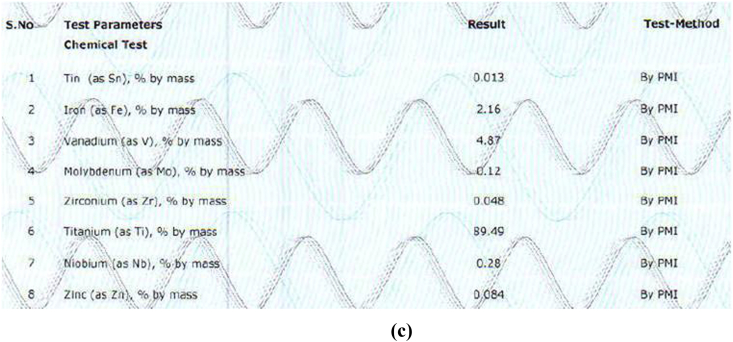
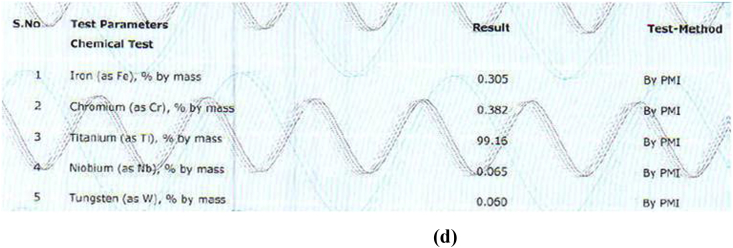
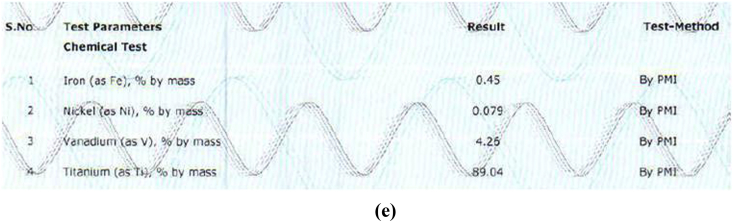
The alloy compositions of different samples obtained from PMI were shown in Fig. 3a, Fig. 3b, Fig. 3c, Fig. 3d, Fig. 3ea,b,3c,3d,3e. The result obtained was tabulated in Table 1. The result obtained by PMI testing showed highest titanium content in sample 2 (Myriad) as 299.47% by mass and least in sample 5(Alpha dent) as 89.04% by mass. Nickel was found only in sample 5(Alpha dent) as 0.079% by mass and zinc in sample 4 (MIS) as 0.084% by mass. Chromium was found in sample 1 (MK III) as 0.263% by mass and in sample 2 as 0.273% by mass. Tungsten was found only in sample 4 as 0.060% by mass. Highest iron content was found in sample 3 as 2.13% by mass and lowest in sample 2 as 0.233% by mass. Tin was found only in sample 3 as 0.013% by mass.
Fig. 3a.
Composition of Sample 1 derived by PMI.
Fig. 3b.
Composition of Sample 2 derived by PMI.
Fig. 3c.
Composition of Sample 3 derived by PMI.
Fig. 3d.
Composition of Sample 4 derived by PMI.
Fig. 3e.
Composition of Sample 5 derived by PMI.
Table 1.
Mean of elemental compositions of different implant samples obtained using PMI testing.
| Composition (% by mass) | Sample 1 (Mk III) | Sample 2 (Myriad) | Sample 3 (Nobel Replace) | Sample 4 (MIS Seven) | Sample 5 (Alpha dent) |
|---|---|---|---|---|---|
| Titanium | 99.32 | 99.47 | 89.49 | 99.16 | 89.04 |
| Iron | 0.262 | 0.233 | 2.16 | 0.305 | 0.45 |
| Manganese | 0.133 | – | – | – | – |
| Chromium | 0.263 | 0.273 | – | 0.382 | – |
| Tin | – | – | 0.013 | – | – |
| Vanadium | – | – | 4.17 | – | 4.26 |
| Molybdenum | – | – | 0.12 | – | – |
| Zirconium | – | – | 0.048 | – | – |
| Niobium | – | – | 0.28 | 0.065 | – |
| Zinc | – | – | 0.084 | – | – |
| Tungsten | – | – | – | 0.060 | – |
| Nickel | – | – | – | – | 0.079 |
4. Discussion
Although titanium is considered as highly biocompatible material but still under certain conditions titanium from dental implants, is released in the presence of oral fluids and tissues. Many studies had showed that Ti either in pure form or as alloys or as titanium oxide nano-particles cannot penetrate skin barrier, however, there are evidence of penetration of Ti through the mucosa of the oral cavity. Metallic biomaterials including titanium when gets degraded causes hypersensitivity reactions, resulting in fatigue, malaise, dull pain, rashes on the skin and even loss of implants in certain cases. Data lacks on the incidence of allergic reactions related to titanium, even though it is a major growing concern today.13
Wennerberg et al.,14 stated that the dental implants made of titanium when placed can cause internal exposure, and in tissues surrounding the implants and regional lymph nodes as well as pulmonary tissue showed presence 100–300 ppm concentration of titanium. A study by Sicilia et al.,15 found that Ti allergy due to dental implants can be detected in patients with a low prevalence 0.6%. In a study by Egusa et al.,10 in their clinical report demonstrated the occurrence of facial eczema due to titanium dental implant placed for implant supported mandibular overdenture. Complete remission from allergic reactions was achieved by when the titanium implant was removed. Hosoki et al.,16 in their study find a 69-year-old male with eczema after orthopedic surgery. After one year the titanium screws placed during the orthopaedic surgery were removed but still eczema persist. The eczema resolves completely once the dental implant of the patient was removed.
In case of dental implants more complex immune reactions may develop around the implants, which lead to inflammation, pain and loosening of implants. The most common metals causing cutaneous and extracutaneous allergies are nickel, cobalt, and chromium. Laine et al.,17 done a study on 118 patients having lichenoid lesions in their mouth, the result showed that 80 patients had type IV allergy to one or more metals.78 patients were allergic to mercury, 17 to nickel, 11 to gold, 4 to cobalt, 3 to tin, 2 to palladium and 1 to chromium. In a study by Waroquier et al.,18 it has been shown that the patient wearing removable resin prosthesis had pruritus over geographic tongue and burning sensations. Type IV allergy to cobalt had been diagnosed by a patch test. Once the clasp from prosthesis was removed, the signs and symptoms of allergy had disappeared. In another study19 patient allergic to nickel shows clinical signs and symptoms of burning sensation, numbness on lateral border of tongue and gingival hyperplasia. Patch test with 5% nickel sulphate confirms the final diagnosis. There was frequent association of nickel allergy with chromium and cobalt allergy. Duarte et al.,20 in their study when tested 1208 patients with contact dermatitis, found that 18.5% patients were allergic to two or three metals.
In the present study a Positive material identification machine was used to analyse the compositional variation found in different commercially available implants and an attempt was done to co-relate the obtained results with allergic conditions caused by various metals. PMI machine contains low radioactive isotopes or x-ray tubes and the exposed material reflects the radiation, generating energy, this reflection will generate a different energy level for every element. This energy is measured and detected, thus identifying the elements present in the alloy.21 In different implant samples highest titanium content was found in sample 2 (Myriad, 99.47%) and least in sample 5(Alpha dent, 89.04%). Alloys like nickel was only found in sample 5 (Alpha dent, Ni-0.079%). Small amount of nickel in titanium alloys may initiate or intensify allergic reactions due to thinning of the stratum corneum which leads to increased absorption of nickel.22 Zinc was found only in sample 4 (MIS) as 0.084% by mass. Dental restorations containing zinc can induce macuopapular rash, oral lichen planus and palmoplantar pustulosis.
Elements present in titanium alloys of dental implant could cause allergic reactions to beryllium, cobalt, chromium, copper, iron, nickel and palladium.7 Kim et al.,23 evaluated the clinical association of oral diseases with contact allergy to dental materials. They found that the most common allergens were nickel sulphate and gold sodium thiosulfate as 25.0% followed by potassium dichromate as 22.7%, cobalt as15.9%, palladium as 6.8%, mercury as 4.5%, copper as 4.5%, and methyl hydroquinone as 4.5%.The reason for this allergic reactions may be metals in their ionic form can bond with native proteins and form haptenic antigens and can activate the degranulation of mastocytes and basophiles, and may lead to type I or type IV hypersensitive reactions.24 It is recommended carrying out metal allergy test for each patient before implant placement and composition of all commercially available implant system must be mentioned on the box.
5. Conclusion
Apart from titanium different other metal contents such as nickel, chromium, zinc etc found in various commercially available implants and this may be allergic to some patients. This study recommends that implant composition should be made mandatory to be disclosed on the implant packet so that an implantologist must be well aware of the material composition of the particular implants. Before placing implant in a patient with known allergy patch test for the allergen present in the particular implant can be done for the patient's health benefit and long term clinical success of dental implants.
Funding
None.
Conflicts of interest
None.
Acknowledgement
None.
Biographies
Avadhesh Kumar Choubey had worked for the concept of the work and acquisition of the data required. He had drafted the paper and responsible for the final approval of the version of the article to be published. He agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any parts of the work are appropriately investigated and resolved.
Sunil Kumar Mishra had designed and interprets the data for the work and drafted the paper along with Avadhesh. He is also responsible for the final approval of the version of the article to be published. He agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any parts of the work are appropriately investigated and resolved.
Ramesh Chowdhary had designed and drafted the article along with Sunil Mishra and revised it critically He is responsible for the final approval of the version of the article to be published. He agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any parts of the work are appropriately investigated and resolved.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jobcr.2019.05.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bruschi M., Nethl D.S., Goriwoda W., Rasse M. Composition and modifications of dental implant surfaces. J Oral Implants. 2015;2015:1–14. [Google Scholar]
- 2.Parithimarkalaignan S., Padmanabhan T.V. Osseointegration: an update. J Indian Prosthodont Soc. 2013;13:2–6. doi: 10.1007/s13191-013-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naveau A., Shinmyouzu K., Moore C., Avivi-Arber L., Jokerst J., Koka S. Etiology and measurement of peri-implant crestal bone loss (CBL) J Clin Med. 2019;8:1–20. doi: 10.3390/jcm8020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh A., Ramachandra K., Devarhubli A.R. Evaluation and comparison of shear bond strength of porcelain to a beryllium-free alloy of nickel-chromium, nickel and beryllium free alloy of cobalt-chromium, and titanium: an in vitro study. J Indian Prosthodont Soc. 2017;17:261–266. doi: 10.4103/jips.jips_337_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goutam M., Giriyapura C., Mishra S.K., Gupta S. Titanium allergy: a literature review. Indian J Dermatol. 2014;59:630. doi: 10.4103/0019-5154.143526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasconselos D.M., Santos S.G., Lamghari M., Barbosa M.A. The two faces of metal ions: from implant rejection to tissue repair/regeneration. Biomaterials. 2016;84:262–275. doi: 10.1016/j.biomaterials.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 7.Vijayaraghavan V., Sabane A.V., Tejas K. Hypersensitivity to titanium: a less explored area of research. J Indian Prosthodont Soc. 2012;12:201–207. doi: 10.1007/s13191-012-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carcuac O., Abrahamsson I., Albouy J.P., Linder E., Larsson L., Berglundh T. Experimental periodontitis and peri-implantitis in dogs. Clin Oral Implant Res. 2013;24:363–371. doi: 10.1111/clr.12067. [DOI] [PubMed] [Google Scholar]
- 9.Miron R.J., Zohdi H., Fujioka-Kobayashi M., Bosshardt D.D. Giant cells around bone biomaterials:Osteoclasts or multi-nucleated giant cells? Acta Biomater. 2016;46:15–28. doi: 10.1016/j.actbio.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Egusa H., Ko N., Shimazu T., Yatani H. Suspected association of an allergic reaction with titanium dental implants: a clinical report. J Prosthet Dent. 2008;100:344–347. doi: 10.1016/S0022-3913(08)60233-4. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi T.P. An overview of the corrosion aspect of dental implants (titanium and its alloys) Indian J Dent Res. 2009;20:91–98. doi: 10.4103/0970-9290.49068. [DOI] [PubMed] [Google Scholar]
- 12.Williams D.F. Definitions in Biomaterials, Proceedings of a Consensus Conference of the European Society for Biomaterials, Chester, England, March 3-5,1986. Elsevier; Amsterdamn: 1987. European society for biomaterials. [Google Scholar]
- 13.Siddiqi A., Payne A.G., De Silva R.K., Duncan W.J. Titanium allergy: could it affect dental implant integration? Clin Oral Implant Res. 2011;22:673–680. doi: 10.1111/j.1600-0501.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- 14.Wennrberg A., Ide-Ektessabi A., Hatkamata S. Titanium release from implants prepared with different surface roughness. Clin Oral Implant Res. 2004;15:505–512. doi: 10.1111/j.1600-0501.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 15.Sicilia A., Cuesta S., Coma G. Titanium allergy in dental implant patients: a clinical study on 1500 consecutive patients. Clin Oral Implant Res. 2008;19:823–835. doi: 10.1111/j.1600-0501.2008.01544.x. [DOI] [PubMed] [Google Scholar]
- 16.Hosoki M., Nishigawa K., Miyamoto Y., Ohe G., Matsuka Y. Allergic contact dermatitis caused by titanium screws and dental implants. J Prosthodont Res. 2016;6 0:213–239. doi: 10.1016/j.jpor.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Laine J., Kalimo K., Happonen R.P. Contact allergy to dental restorative materials in patients with oral lichenoid lesions. Contact Dermatitis. 1997;36:141–146. doi: 10.1111/j.1600-0536.1997.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 18.Waroquier D., Evrard L., Nelis M., Parent D. Allergic contact dermatitis presenting as geographical tongue with pruritus. Contact Dermatitis. 2009;60:106–119. doi: 10.1111/j.1600-0536.2008.01352.x. [DOI] [PubMed] [Google Scholar]
- 19.Noble J., Ahing S.I., Karaiskos N.E., Wiltshire W.A. Nickel allergy and orthodontics, a review and report of two cases. Br Dent J. 2008;204:297–300. doi: 10.1038/bdj.2008.198. [DOI] [PubMed] [Google Scholar]
- 20.Duarte I., Amorim J.R., Perázzio E.F., Schmitz Junior R. Metal contact dermatitis: prevalence to nickel, cobalt and chromium. An Bras Dermatol. 2005;80:137–142. [Google Scholar]
- 21.Skocaj M., Filipic M., Petkovic J., Novak S. Titanium dioxide in our everyday life: is it safe? Radiol Oncol. 2011;45:227–247. doi: 10.2478/v10019-011-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuh A., Thomas P., Kachler W. Allergic potential of titanium implants. Orthopade. 2005;34:327–328. doi: 10.1007/s00132-005-0764-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim T.W., Kim W.I., Mun J.H. Patch testing with dental screening series in oral disease. Ann Dermatol. 2015;27:389–393. doi: 10.5021/ad.2015.27.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forte G., Petrucci F., Bocca B. Metal allergens of growing significance: epidemiology, immunotoxicology, strategies for testing and prevention. Inflamm Allergy - Drug Targets. 2008;7:145–162. doi: 10.2174/187152808785748146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.