Abstract
Thoracic endovascular aortic repair (TEVAR) provides an alternative to open surgery for a variety of aortic diseases. However, complex anatomy and previous operations may preclude traditional approaches to TEVAR. Percutaneous transapical access through the left ventricle is a feasible option to facilitate externalized “rail” wire support for complex TEVAR. We present the case of TEVAR for a residual type B aortic dissection facilitated by percutaneous transapical access.
Keywords: Vascular closure, Thoracic endovascular aortic repair, Transapical access
Thoracic endovascular aortic repair (TEVAR) provides multiple advantages compared with open repair for a variety of aortic diseases.1, 2 However, complex aortic anatomy may make TEVAR technically challenging. For example, TEVAR is often used for management of residual type B aortic dissection after surgical repair of an acute type A dissection. In this setting, it may be difficult to gain wire access to the true lumen or to advance equipment safely into the ascending aortic graft. In these cases, advanced techniques including upper extremity access have been used to facilitate wiring. In some cases, snaring and externalizing a wire to create a brachial-femoral rail is necessary to provide enough support to deliver the endograft.
Percutaneous transapical (TA) access has been used in cardiology procedures for >50 years. Needle TA puncture of the left ventricle was originally performed to measure left ventricular pressure in the presence of mechanical aortic and mitral valves.3 Interventional TA access has traditionally been achieved with direct surgical exposure of the left ventricular apex through a minithoracotomy. More recently, smaller bore percutaneous access of the left ventricular apex has been described for interventional and electrophysiology procedures.4, 5, 6 Percutaneous TA access is now most commonly used for structural interventions involving the mitral valve or as an alternative to femoral access in patients with severe peripheral artery disease.
We present a multidisciplinary case of complex TEVAR accomplished with the assistance of TA access for antegrade wiring of a complex type B aortic dissection. The patient provided written consent to share the case and the case images.
Case report
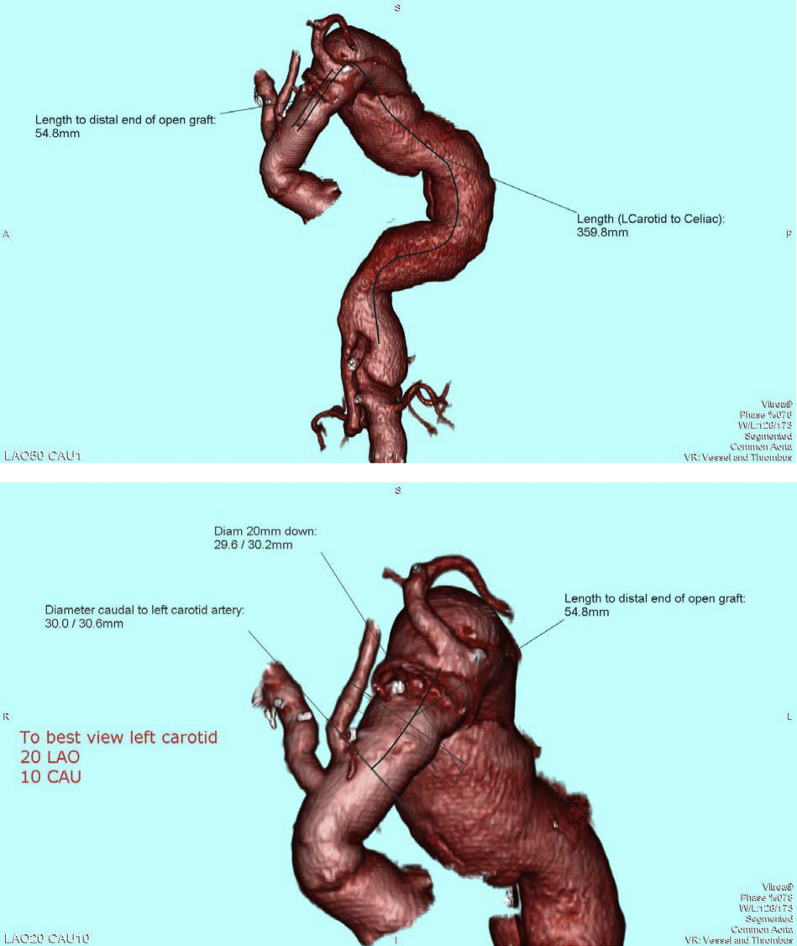
A 61-year-old man with a remote history of type A aortic dissection managed with a modified Bentall procedure subsequently developed aneurysmal degeneration of the aortic arch and descending aorta and a type B dissection. A two-stage surgical approach was planned. Open total arch replacement was performed first for correction of the ascending aortic aneurysm. This was followed by planned TEVAR of aortic dissection (Fig 1).
Fig 1.
Reconstruction of preoperative computed tomography image of aorta demonstrating aortic arch reconstruction, severe aortic tortuosity, and type B aortic dissection with aneurysmal dilation of the thoracic and abdominal aorta.
An initial attempt at TEVAR failed because of the complex anatomy. Specifically, attempts to advance a guidewire from a femoral approach into the aortic graft failed because of severe tortuosity and a large false lumen. Therefore, right brachial access was obtained, and a guidewire was advanced from the brachial artery through the aortic root graft and into the true lumen of the descending aorta. This wire was snared and externalized through a femoral artery sheath. However, despite exchange for a supportive wire and a femoral-brachial rail, the endovascular graft was not able to be advanced into the appropriate position. Specifically, because of the prior ascending arch reconstruction and surgical debranching, the traditional brachial-femoral wire approach did not allow the TEVAR device to be placed sufficiently proximal into the surgical graft to have an adequate seal zone. Therefore, a second attempt at TEVAR was planned, this time with TA access assistance.
Before TA puncture, bifemoral arterial access was obtained by cutdown on the right and in a percutaneous fashion on the left. Percutaneous left femoral venous access was obtained. After the induction of anesthesia, the anesthesiologist placed a dual-lumen airway. The left lung was then deflated to allow optimal positioning of the left ventricular apex. Single-lung ventilation was continued. The left ventricular apex was palpated in the left fifth intercostal space, in the midclavicular line. Transthoracic echocardiography (TTE) was used to confirm the location of the apex, and a marker was placed at this site. Left coronary angiography was performed with the marker in place to ensure that the course of the left anterior descending coronary artery was not near the planned apical puncture site (Fig 2). Apical puncture was performed with a 21-gauge, 7-cm micropuncture needle with TTE guidance and with the left lung deflated. A standard micropuncture wire was advanced into the left ventricle, and the needle was exchanged for a micropuncture sheath. Location was confirmed with pressure tracing and a limited ventriculogram. A standard 5F sheath was then placed into the left ventricular cavity over a standard 0.035-inch wire and sutured in place to prevent movement. Therapeutic anticoagulation was then administered.
Fig 2.
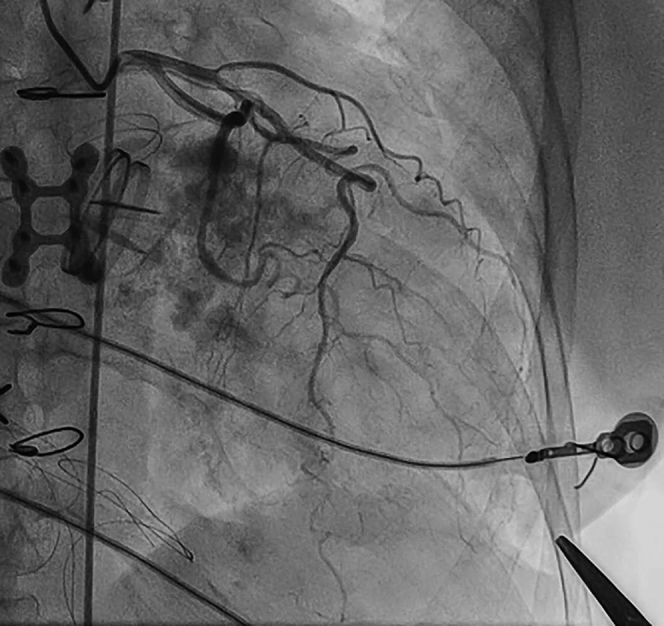
Coronary angiography before transapical (TA) puncture. Once the apex is identified by palpation and transthoracic echocardiography (TEE), a marker (hemostat) is placed over the left ventricular apex and coronary angiography is performed. If the course of the left anterior descending artery is adequately distant from the marker, it is safe to proceed with percutaneous TA puncture.
A 0.035-inch curved Glidewire Advantage guidewire (Terumo Interventional Systems, Tokyo, Japan) was inserted through the apical sheath and advanced across the mechanical aortic valve and aortic arch graft. Fluoroscopy confirmed that the wire was not impeding motion of the aortic valve leaflets, which is a risk of traversing a mechanical valve with a guidewire. In addition, TTE was used to ensure that there was no significant aortic insufficiency or a dramatic change in left ventricle size. The wire was then snared in the descending thoracic aorta and externalized through the right common femoral artery sheath. This wire was exchanged for an Amplatz Extra-Stiff guidewire (Cook Medical, Bloomington, Ind) to facilitate a through-and-through rail wire from the right femoral sheath to the apical sheath.
Reconstruction of the aortic arch and descending thoracic aorta was then performed with delivery of three endovascular grafts (Valiant Captivia; Medtronic, Santa Rosa, Calif) through the right femoral artery approach (Fig 3). The right femoral arteriotomy was repaired with 5-0 Prolene suture.
Fig 3.
Thoracic endovascular aortic repair (TEVAR). After antegrade wiring of the true lumen from the transapical (TA) sheath, the wire was externalized to create a through-and-through rail (A). The endograft was delivered from the right femoral artery sheath (B). After stent deployment, aortography (C) reveals an excellent repair with demonstration of TA access site (*).
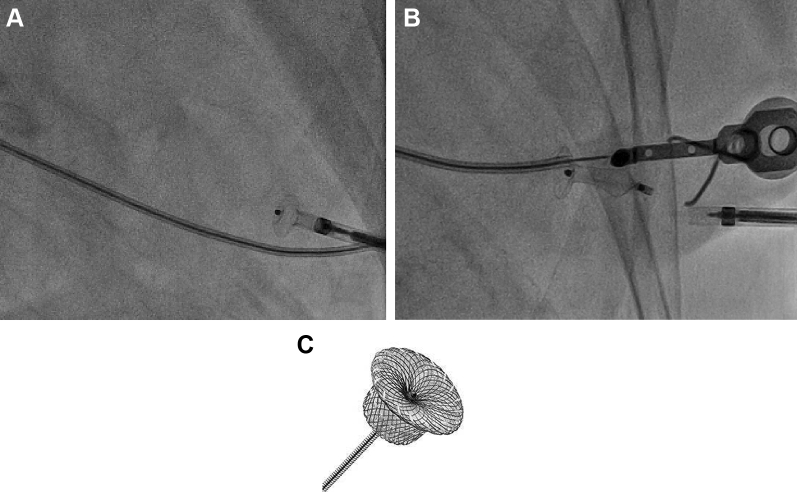
The TA site was closed with a 6/4-mm Amplatzer Duct Occluder (Abbott Vascular, Santa Clara, Calif; Fig 4). Repeated coronary angiography was performed to confirm patency of the left anterior descending coronary artery.
Fig 4.
Closure of percutaneous transapical (TA) puncture site with occluder device. Closure of the TA access site is achieved with an Amplatzer Duct Occluder (C; Abbott Vascular, Santa Clara, Calif) by deploying the device in the apical left ventricle and pulling the disk against the apical wall (A). Once adequate seal of the left ventricular apex is achieved, the device is released (B). Routine ventriculography is then performed to confirm adequate closure of the ventriculotomy.
Discussion
The interventional cardiology literature has relatively few reports addressing the safety and utility of interventional percutaneous TA access. Reported complications include cardiac tamponade, pneumothorax, and hemothorax. Higher rates of complications have been reported with manual hemostasis, including one report citing an overall complication rate of 62%.5 However, when the TA access site is closed with a device, the rate of complications is lower. Jelnin et al4 previously reported outcomes of a 26-patient cohort who underwent structural interventions through the TA approach. In this cohort, TA access was achieved with the assistance of cardiac computed tomography angiography for intraprocedural guidance of apical anatomy and the “safe puncture window.” A range of delivery sheaths were used (5F to 12F). Manual pressure was held on the 5F access sites. Twenty of the 22 sites >5F were closed with an Amplatzer Duct Occluder. Two patients in the overall cohort had procedure-related complications (7.1%). It seems that routine closure of the TA puncture site is safer than manual hemostasis. Our standard technique for TA access includes routine coronary angiography to ensure that the puncture site will not compromise the coronary vasculature. We also ask the anesthesiologist for solitary right-lung ventilation. The anesthesiologists at our center are adept in this technique, and we think that it reduces the risk of lung injury at the time of TA puncture.
A number of closure devices designed for percutaneous TA closure are under investigation, some with CE mark.7, 8, 9 These devices achieve TA closure in various ways, including sutured and suture-less techniques. None of the TA closure devices are currently available in the United States. Therefore, our standard practice is to use the Amplatzer Duct Occluder 6/4 for closure of the TA site (Fig 4). We use the same size occluder device for all small-bore (<9F) TA punctures. The “cap” of the device is 10 mm. This cap is pulled back to the wall of the left ventricular apex. The “6” in the size refers to the diameter in millimeters of the device below the cap, and the “4” refers to the size of the tapered distal end in millimeters. The device is 7 mm long. It is designed for closure of a patent ductus arteriosus. When the device is used for this purpose, it is delivered from the pulmonary artery across the patent ductus arteriosus and into the aorta. The cap is then deployed in the aorta and pulled back against the aortic wall. The rest of the device is pulled back until the 4-mm portion of the device is deployed in the pulmonary artery. When it is used for the TA puncture site, the cap of the device rests in the left ventricular cavity and is pulled back against the apical wall. The waist of the device elongates to fill the track created by the sheath through the myocardium. The precise size of the hole in the myocardial wall is difficult to estimate because the puncture is performed while the apex is in motion. Therefore, we have elected to use the 6/4 size device. We have closed many TA punctures with this method and have experienced >90% success with this technique.
This case is an example of a multidisciplinary team effort. The interventional cardiology service performed the TA puncture and the coronary angiography. The vascular surgery service performed the TEVAR, snaring, and femoral cutdown. As in any high-risk procedure, the authors encourage collaboration across disciplines to ensure that the specific skill sets of each physician are used to provide individualized, safe, and effective care for patients.
Conclusions
In cases with difficult anatomy, percutaneous TA access may be used to facilitate wiring and support during complex TEVAR. Percutaneous closure of the apical site may be accomplished with off-label use of an Amplatzer Duct Occluder 6/4 mm.
Footnotes
Author conflict of interest: A.P.S. is a proctor and consultant for Abbott Vascular. R.M. is a consultant for Medtronic and W. L. Gore.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Gopaldas R.R., Huh J., Dao T.K., Lemaire S.A., Chu D., Bakeen F.G. Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients. J Thorac Cardiovasc Surg. 2007;140:1001–1010. doi: 10.1016/j.jtcvs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Conrad M.F., Ergul E.A., Patel V.I., Parachuri V., Kwolek C.J., Cambria R.P. Management of diseases of the descending thoracic aorta in the endovascular era. Ann Surg. 2010;252:603–610. doi: 10.1097/SLA.0b013e3181f4eaef. [DOI] [PubMed] [Google Scholar]
- 3.Brock R., Milstein B.B., Ross D.N. Percutaneous left ventricular puncture in the assessment of aortic stenosis. Thorax. 1956;163:163–171. doi: 10.1136/thx.11.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jelnin V., Dudiy Y., Einhorn B.N., Kronzon I., Cohen H.A., Ruiz C.E. Clinical experience with percutaneous left ventricular transapical access for interventions in structural heart defects: a safe access and secure exit. JACC Cardiovasc Interv. 2011;4:868–874. doi: 10.1016/j.jcin.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Pitta S.R., Cabalka A.K., Rihal C.S. Complications associated with left ventricular puncture. Catheter Cardiovasc Interv. 2010;76:993–997. doi: 10.1002/ccd.22640. [DOI] [PubMed] [Google Scholar]
- 6.Vurgun V.K., Altin A.T., Kilickap M., Candemir B., Akyurek O. Percutaneous transapical approach and transcatheter closure for ventricular tachycardia ablation. Pacing Clin Electrophysiol. 2018;41:334–337. doi: 10.1111/pace.13213. [DOI] [PubMed] [Google Scholar]
- 7.Ziegelmueller J.A., Lange R., Bleiziffer S. Access and closure of the left ventricular apex: state of play. J Thorac Dis. 2015;7:1548–1555. doi: 10.3978/j.issn.2072-1439.2015.09.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenstein J., Kempfert J., Van Linden A., Arsalan M., Mollmann H., Kim W. Sutureless transapical access and closure to facilitate transapical transcatheter aortic valve implantation: first-in-human use. J Am Coll Cardiol. 2013;62:763. doi: 10.1016/j.jacc.2013.02.096. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari E., Demertzis S., Angelella J., Berdajs D., Tozzi P., Moccetti T. Apical closure device for full-percutaneous transapical valve implantation: stress-test in an animal model. Interact Cardiovasc Thorac Surg. 2017;24:721–726. doi: 10.1093/icvts/ivw433. [DOI] [PubMed] [Google Scholar]