FIG 7.
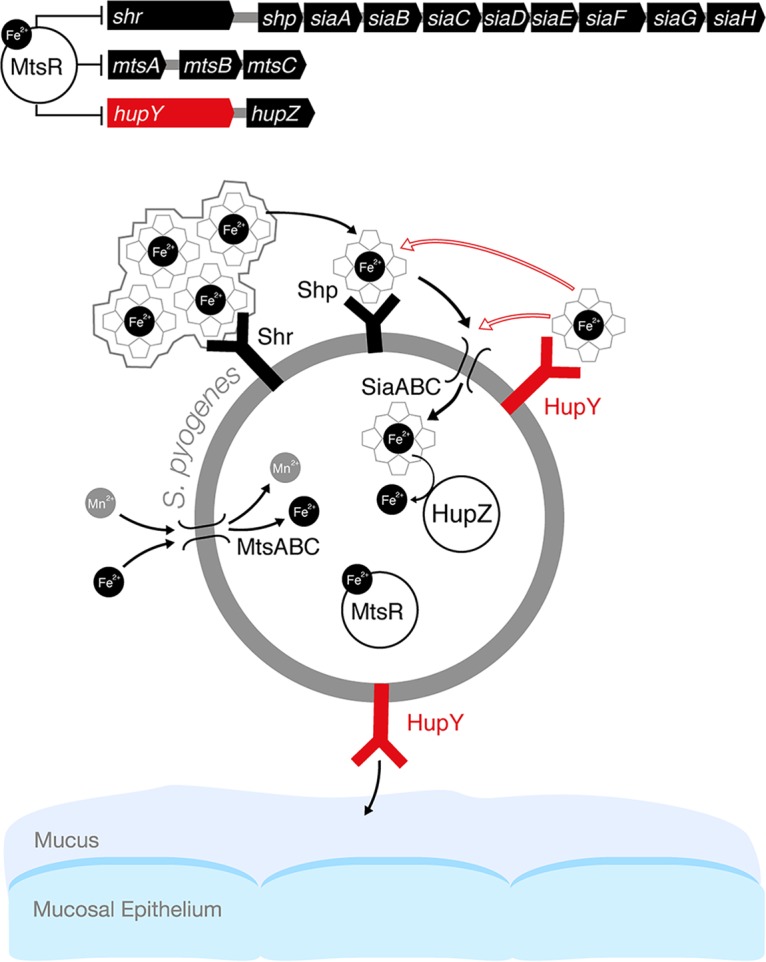
Model of the role of HupY in GAS colonization and heme utilization on mucosal surfaces. In the presence of high levels of iron, iron-bound MtsR represses a large number of genes, including the sia operon, which includes the genes for the siaABC heme importer as well as shr, and shp heme binding proteins. In addition, MtsR represses the manganese and iron mtsABC transporter, hupZ, and hupY. MtsR also downregulates the mtsABC gene cluster in a manganese-dependent manner (32; not shown in the model). Under iron-depleted conditions such as on mucosal surfaces, MtsR repression is relieved, leading to upregulation of these operons. Shr captures heme (from host hemoproteins or the environment) and delivers it to Shp and subsequently to SiaABC for import into the cell. Once inside the cell, iron can be liberated from heme by the HupZ enzyme. HupY, coregulated with HupZ, is a surface protein that has the ability to bind heme. We propose that HupY binds heme to allow transport to HupZ, either through the Shp/SiaABC heme import pathway or through another mechanism. Open arrows indicate possible heme transfer pathways. HupY also plays a role in mucosal colonization, possibly as an adhesin important for binding to host cells.
