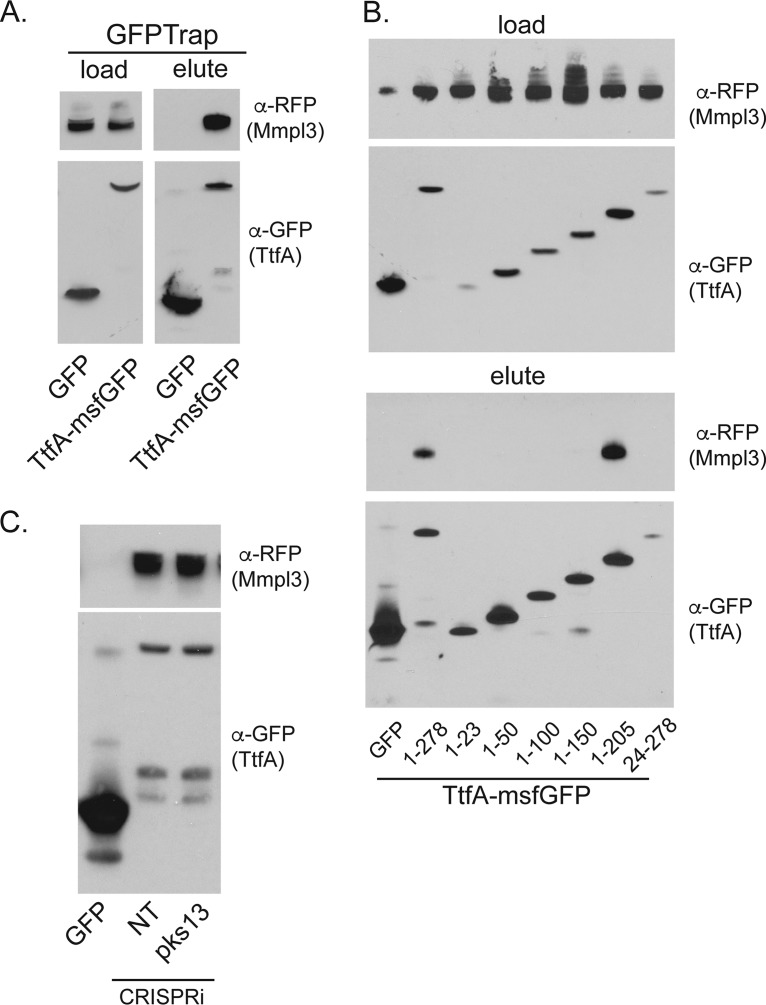
FIG 4.
TtfA and MmpL3 form a complex in vivo via the essential region of TtfA and independently of TMM synthesis. (A) DDM-solubilized M. smegmatis lysates (left) and GFP-Trap eluates (right) of msfGFP-expressing control (MGM6828) and TtfA-msfGFP (MGM6815) both coexpressing MmpL3-mCherry and probed with anti-RFP (top) and anti-GFP (bottom). (B) The essential region of MsTtfA is necessary and sufficient for MmpL3 interaction. DDM-solubilized lysates (top) and GFP-Trap eluates (bottom) of msfGFP control (MGM6828), full-length TtfA-msfGFP (1-278 [MGM6829]), or TtfA-msfGFP truncations (1-23 [MGM6826], 1-50 [MGM6823], 1-100 [MGM6827], 1-150 [MGM6824], 1-205 [MGM6822], 24-278 [MGM6825]) coexpressing MmpL3-mCherry and probed with anti-RFP (top) and anti-GFP (bottom). (C) The MsTtfA-MmpL3 interaction is independent of mycolate synthesis. GFP-Trap eluates of MmpL3-mCherry expression strains coexpressing msfGFP control (MGM6828) or TtfA-msfGFP with either control CRISPRi (NT [MGM6816]) or Pks13 depleted (MGM6817) for 6 h with ATc. Top panel is probed for MmpL3-mCherry with anti-RFP and bottom with anti-GFP.