Abstract
Background
Resolution and prevention of peri‐implant mucositis are a key in preventing peri‐implantitis. This case–control study aims to assess the modifying effect of a deep mucosal tunnel (DMT) on the induction and resolution phases of experimental peri‐implant mucositis.
Methods
Nineteen subjects with a tissue level implant were assigned to cases (DMT, depth ≥3 mm) or controls (shallow mucosal tunnel ≤1 mm, SMT). Subjects underwent a standard experimental peri‐implant mucositis protocol characterized by an oral hygiene optimization phase, a 3‐week induction phase using an acrylic stent to prevent self‐performed oral hygiene at the experimental implant, and a 3 + 2 weeks resolution phase. Modified plaque (mPI), gingival index (mGI) and peri‐implant sulcus fluid IL‐1β concentrations were measured over time. Differences between DMT and SMT were assessed with the Mann–Whitney test.
Results
Modified plaque index and mGI increased in parallel during the induction phase. After resumption of oral hygiene practice, mPI and mGI resolved towards baseline values in the SMT group. In DMT, mPI and mGI values diverged: plaque resolved but resolution of inflammation was delayed and of smaller magnitude during the first 3 weeks after resumption of oral hygiene. IL‐1β concentrations were significantly higher in DMT at 21 days (end of induction) and during the resolution phase corroborating the clinical findings. Removal of the crown and submucosal professional cleaning were needed to revert mGI to baseline values in DMT implants.
Conclusions
The depth of the mucosal tunnel modifies the resolution of experimental peri‐implant mucositis at transmucosal implants. This observation raises important questions on the effectiveness of self‐performed oral hygiene in cases where implants are placed deeper and the ability to resolve mucositis and effectively prevent peri‐implantitis in such situations.
Keywords: case–control study, dental implant, experimental gingivitis, mucositis prevention, peri‐implant mucositis, peri‐implantitis prevention, transmucosal implant
1. INTRODUCTION
Peri‐implant mucositis is defined as soft tissue inflammation around an osseointegrated dental implant in the absence of continuous marginal peri‐implant bone loss (Berglundh et al. 2018). It is considered to be the precursor of peri‐implantitis (Heitz‐Mayfield & Salvi, 2018) and therefore a target for prevention (Jepsen et al., 2015). Twenty‐five years of research, using the application to dental implants of the well established experimental gingivitis protocol (Loe, Theilade, & Jensen, 1965), have established a cause and effect relationship between plaque accumulation around implants and the onset of peri‐implant mucositis (Meyer et al., 2017; Pontoriero et al., 1994; Salvi et al., 2012; Schincaglia et al., 2017; Zitzmann, Berglundh, Marinello, & Lindhe, 2001).
The experimental peri‐implant mucositis study performed by Salvi et al. (2012) has indicated that plaque accumulation at tissue level dental implant sites with epi‐mucosal implant platform leads to changes in local biofilm and inflammation in humans. Following resumption of oral hygiene, inflammation resolved, but interestingly the gingival index and inflammatory biomarkers remained higher than baseline after 3 weeks of resumption in oral hygiene (Salvi et al., 2012).
Accessibility for biofilm removal around implant prosthesis is critical for preventing and managing peri‐implant diseases (Heitz‐Mayfield & Salvi, 2018; O'Mahony, Macneill, & Cobb, 2000; Pontoriero et al., 1994).
A randomized clinical trial on the management of peri‐implant mucositis showed that implants with supra‐mucosal restoration margins yielded significantly greater reductions in probing depths following treatment of peri‐implant mucositis compared with those with submucosal restoration margins (Heitz‐Mayfield et al., 2011).
A clinical retrospective study also showed that high proportions of implants diagnosed with peri‐implantitis were associated with inadequate biofilm control or lack of accessibility for oral hygiene measures, while peri‐implantitis was rarely detected at implants with cleansable prosthesis or when proper biofilm control was performed (Serino & Strom, 2009). Emerging evidence indicates that the location and geometry of the implant platform is associated with biological complications (Derks et al., 2016a; Katafuchi, Weinstein, Leroux, Chen, & Daubert, 2018).
In order to obtain aesthetically pleasing results, however, implants are frequently placed deeper into the soft tissues to mask the metal components and provide adequate space for the development of an ideal tooth form before the emergence of the crown from the soft tissue margin. This creates a mucosal tunnel between the implant–prosthesis interface and the soft tissue margin. In another word, the implant–prosthesis interface is subgingival.
As oral hygiene practices have only limited effect below the soft tissue margins into periodontal pockets (Waerhaug, 1981), the depth of the mucosal tunnel is a possible effect modifier of such preventive and treatment strategies. Indeed, significant evidence indicates that normal preventive care has limited effect on the resolution of peri‐implant mucositis and its limited effectiveness may contribute to the risk of developing peri‐implantitis (Costa et al., 2012).
No study, so far, has investigated the effect of the depth of the mucosal tunnel on the onset and resolution of experimental peri‐implant mucositis in humans. The aim of this case–control study was to determine whether the depth of the mucosal tunnel has an effect on the development and resolution of experimental peri‐implant mucositis.
2. MATERIALS AND METHODS
2.1. Patient population and study design
This was a prospective clinical study, with subjects (each with at least one transmucosal implant—tissue level platform—with a screw‐retained restoration and no evidence of marginal alveolar bone loss) undergoing a modified experimental peri‐implant mucositis protocol over an 84‐day (12 weeks) period. The study design is illustrated in Figure 1. The Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster approved the study protocol (Approval number: UW 17‐524). Written consent form was obtained from all the subjects before the study. The study was prospectively registered with the Honk Kong Clinical Trial Registry (Registration ID HKUCTR‐2317). All clinical study procedures were performed between March and July 2018.
Figure 1.
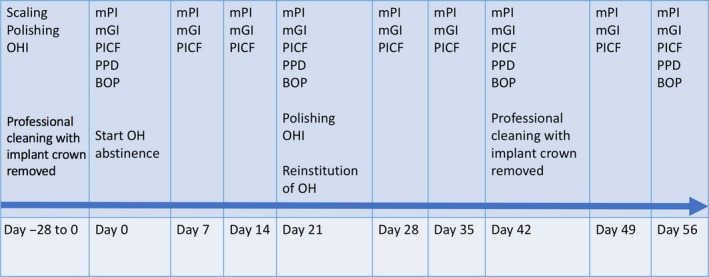
Schematic illustration of experimental design and procedures
Potential participants were identified from the database of subjects who received dental implants at Prince Philip Dental Hospital and screened for eligibility by a single investigator in the Periodontal Clinics of the Faculty of Dentistry, University of Hong Kong (PPDH). Only transmucosal implants (Straumann tissue level implants) with screw‐retained restorations and without radiographic signs of alveolar bone loss were considered. Subjects had to fulfil the following inclusion criteria: 18 years or older, systemically healthy, non‐smoker, healthy or treated periodontal conditions, full‐mouth plaque score (FMPS) ≤25% and full‐mouth bleeding score (FMBS) ≤25% at baseline (before initial plaque control period), width of keratinized tissue ≥2 mm around experimental implant, no use of systemic antibiotics or any other medication known to affect the gingiva or the inflammatory reaction in the preceding 3 months, no need for antibiotic prophylaxis, no history of head and neck radiation, ability and willingness to comply with all study requirements and give informed consent.
Cases were defined as subjects with transmucosal dental implants with a mucosal tunnel with a depth of 3 mm or more (deeper mucosal tunnel, DMT) while controls had a mucosal tunnel depth of 1 mm or less (shallow mucosal tunnel, SMT). Subjects were assigned to the DMT or SMT group through a two‐stage process. Intraoral radiographs were screened to identify reconstructions with the endosteal portion of the implant located apically to the marginal crest of bone in the neighbouring teeth. The depth of the mucosal tunnel, measured from the implant shoulder to the mucosal margin, was confirmed after removal of the crown during the pre‐experimental oral hygiene and prophylaxis phase classifying it as DMT or SMT (Figure 2).
Figure 2.

Diagram illustrating the depth of the mucosal tunnel at implants as assessed after corwn removal. Please note the position of the alveolar crest at the neighbouring teeth and with reference to the endosteal portion of the implant. MT: mucosal tunnel
Subjects underwent an intensive plaque control regimen to optimize oral hygiene and control gingival inflammation in the whole of the dentition followed by a experimental plaque accumulation and resolution essentially as described by Löe et al. (1965). This included removal of screw‐retained restorations to ensure optimal biofilm removal and resolution of inflammation. Normal oral hygiene practices were interrupted at the experimental implant site by instructing the patient to wear an acrylic stent blocking access to the experimental area during daily oral hygiene for 21 days (Schincaglia et al., 2017). Thereafter, volunteers were instructed to resume regular oral hygiene in the experimental area for another 21 days. At completion of this first resolution period, crowns were removed; a professional prophylaxis was performed to ensure complete cleaning of the implant platform into the mucosal tunnel and of the prosthetic components. Subjects were instructed to continue regular oral hygiene and were monitored for an additional 2 weeks.
2.2. Clinical parameters
A single calibrated examiner (DC) took all clinical parameters at six sites around each experimental implant using a UNC 15 periodontal probe. Plaque accumulation was evaluated using the plaque index as adapted for dental implants (modified plaque index [mPI]; Mombelli, van Oosten, Schurch, & Land, 1987). Peri‐implant mucosa inflammation was evaluated using the gingival index as modified for dental implants (modified gingival index [mGI]; Mombelli et al., 1987). mPI and mGI were assessed weekly during the whole experiment by a single calibrated investigator blind to the group assignment. Mean mGI and mPI values were calculated for each implant.
2.3. Peri‐implant crevicular fluid sampling and analysis
Peri‐implant crevicular fluid (PICF) samples were collected for 30 s with sterile paper strips (Periopaper; Oraflow Inc., Smithtown, NY, USA) using the intra‐crevicular method from the mesial‐buccal and disto‐lingual sites of each implant. The sites were first isolated with cotton rolls and a saliva ejector and gently air‐dried. After sampling, PICF volumes were determined non‐destructively using a calibrated Periotron 6000. The Periotron calibration curve was constructed by wetting paper strips with 0.1–1.0 μl of saline measured with a 1.0‐μl Hamilton microsyringe equipped with a Cheney adaptor. Calibration and volume determination were as previously described (Tonetti, Cugini, & Goodson, 1990). Subsequently, the paper strip was placed into a screw‐top plastic vial containing 200 μl of phosphate‐buffered saline (PBS, pH = 7.2) and NaN3 and placed immediately on ice. Paper strips were stored at −80°C until assayed. On the day of analysis, samples were vortexed on ice for 30 s and centrifuged at 3,000 g at 4°C for 5 min. Supernatants were assayed for IL‐1β concentrations using a commercially available high sensitivity ELISA kit (R&D Systems Europe Ltd, Abingdon, UK) according to the manufacturer's instructions. Samples were diluted to be in the linear range of the assay. The detection level of the assay was 0.063 pg/ml.
2.4. Sample size and data analysis
A total sample size of 16 were required in a case–control design to detect a 20% difference in the primary outcome (mGI) between DMT and SMT at 21 and 42 days using the Mann–Whitney U test with alpha set at 0.05 and 80% power based on a median of 1.5 at day 21 and 0.5 at day 42, as previously reported (Salvi et al., 2012; Schincaglia et al., 2017). Data were entered in an excel database, proofed for entry errors and imported into SPSS. Analyses were performed using SPSS version 25. Clinical (mPI, mGI) and host‐derived parameters (IL‐1β) are presented as box plots with median, minimal and maximal values as well as 25% and 75% percentiles, and T‐bars represent the inner fences corresponding to approximately 95% of the observations. Outliers are displayed as asterisks or dots. IL‐1β concentrations have been normalized based on PICF volumes with reference to the Periotron calibration curve. Significance of differences in baseline patient characteristics between DMT and SMT was tested by t test. The null hypothesis of no significant clinical and IL‐1β differences between DMT and SMT during: (a) the 3‐week experimental plaque accumulation (day 0–21); (b) the 3‐week resolution phase after resumption of oral hygiene (day 21–42); and (c) the 2‐week oral hygiene period after professional implant cleaning (day 42–56) was tested calculating the area under the curve (AUC) and using Mann–Whitney U test. Significance of differences at specific time points was tested when the difference in AUC was significant in the specific phase. In such instances to correct for multiple testing, a Bonferroni correction was applied, and significance was set at 0.05.
3. RESULTS
3.1. Study population
Subject recruitment was stopped after reaching the targeted sample size. All 19 subjects (Nine males and 10 females) who accepted to participate in the study completed it. The mean age of the control and test subjects was 56.2 ± 9.7 and 53.7 ± 13.6, respectively (p = 0.64, NS). Eight subjects presented with a history of successfully treated periodontal disease with no residual pockets. Baseline FMPS was 17.2 ± 5.2 and 16.1 ± 3.9 (p = 0.44) and FMBS was 13 ± 5 and 16.7 ± 4.5 (p = 0.77) for SMT and DMT, respectively. All subjects had FMPS and FMBS <25% at baseline. All implants were in the posterior region (five premolars, 14 molars). For the control group, all implants had mucosal tunnel depth of 1 mm or less while in the test group the mucosal tunnel depth was 3 mm or more as assessed after crown removal 28 days before the beginning of the plaque accumulation period. Figure 3 illustrates the location of the alveolar bone crest at the experimental implants with respect to the position of the crest at the neighbouring teeth; it was 3.1 ± 0.7 mm apical to the crest of the neighbouring teeth in the DMT group and 0.5 ± 0.4 mm in the SMT group. The difference was statistically significant (p = 0.043).
Figure 3.

Distance in mm from the bone crest of the adjacent tooth to the most coronal extent of radiographic bone to implant contact for shallow mucosal tunnel (SMT) implants (blue) and deeper mucosal tunnel (DMT) implants (red). This parameter describes the depth of the mucosal tunnel in DMT and SMT. Differences between groups are significant (p = 0.043)
3.2. Experimental plaque accumulation and peri‐implant mucosal inflammation
Figure 4 reports the time course of experimental peri‐implant mucositis. Figure 4a illustrates changes in terms of mPI. No differences between DMT and SMT were observed in the induction, resolution or post‐professional tooth‐cleaning phase (test for differences in AUC, p = 0.905). Onset and resolution of experimental plaque accumulation can be observed indicating the validity of the experiment. Supragingival professional tooth cleaning was performed at day 21, and submucosal instrumentation associated with removal of the screw‐retained crowns was performed at day 42.
Figure 4.

Experimental peri‐implant mucositis time course showing the time course of modified plaque index (mPI), modified gingival index (mGI) and IL‐1b concentrations in (panel a, b and c), respectively. Shallow mucosal tunnel (SMT) is in blue and deeper mucosal tunnel (DMT) is in red. After a 4‐week period of oral hygiene, subjects were instructed to wear a stent during daily oral hygiene to avoid cleaning the experimental implant from day 0 to day 21. At day 21, professional tooth cleaning was performed and patients were instructed to resume normal oral hygiene until day 42. At day 42, crowns were removed and professional tooth cleaning extending in the submucosal area was performed and subjects were again instructed to continue regular oral hygiene practices. Significance of differences in the time courses was analysed as areas under the curve (AUC). No significant difference in AUC (p = 0.905) between DMT and SMT for mPI. Significant differences in AUC between DMT and SMT for mGI and IL‐1β were observed in the resolution phase. Asterisks placed next to the time point indicate significant inter‐group differences between DMT and SMT (Mann–Whitney U test with Bonferroni correction)
Figure 4b reports the time course of the experimental peri‐implant mucosal inflammation in terms of mGI. Onset and resolution of mucosal inflammation can be observed indicating the validity of the experiment. Comparison of the overall time course showed a significant difference between DMT and SMT implants (test for differences in AUC, p = 0.025). No differences in mGI were observed between DMT and SMT during the induction phase up to day 21 (test for differences in AUC, p = 0.549). Highly significant differences, however, were noted comparing DMT and SMT during the resolution phase from day 21 to day 42 with the SMT showing greater and faster resolution of inflammation compared to the DMT (test for differences in AUC, p = 0.003). A significant inter‐group difference was also observed in the 2 weeks following implant crown removal and professional biofilm removal performed at day 42 (test for differences in AUC, p = 0.004).
Figure 4c reports the concentrations of IL‐1β over the course of the experiment. Significant inter‐group differences were observed during the induction phase (test for differences in AUC, p = 0.017), and this could be attributed to higher concentrations of IL‐1β at 21 days (p < 0.001). Significant inter‐group differences were observed during the resolution phase (test for differences in AUC, p = 0.013) with greater concentrations being observed in the DMT group at 28, 35 and 42 days (p < 0.05 after Bonferroni correction). Concentrations after crown removal and professional biofilm removal were also significantly different comparing DMT and SMT (test for differences in AUC, p = 0.035).
Results of a site‐specific analysis aimed at validating IL‐1β concentrations by correlating them with mGI values are displayed in Figure 5. It shows significantly higher IL‐1β concentrations for higher mGI values (Spearman correlation coefficient = 0.579, p < 0.001).
Figure 5.

Box plots of peri‐implant crevicular fluid (PICF) IL‐1β concentrations across modified gingival index (mGI) values (site‐based analysis to validate measurements). A significant correlation between local mGI and IL‐1β concentrations in PICF was observed (Spearman correlation coefficient = 0.579, p < 0.001)
4. DISCUSSION
This study showed that the depth of the mucosal tunnel is a modifier of the resolution of plaque‐induced experimental peri‐implant mucosa inflammation. Data indicate that plaque accumulation, mucosal inflammation and interleukin‐1b concentration in PICF change in a synchronous way in controls with SMTs over the different phases of induction and resolution and confirm the results of previous studies (Salvi et al., 2012). A different pattern was observed at implants with a DMT. While, in general, no significant differences were observed in the induction phase in terms of clinical and biochemical markers of mucosal inflammation, implants with deeper peri‐implant mucosal tunnels displayed a delayed and incomplete resolution of clinical and biochemical markers of inflammation after supra‐mucosal professional cleaning and resumption of self‐performed oral hygiene. Clinical parameters returned to baseline levels in the test group only after removal of screw‐retained crowns and submucosal professional cleaning. Changes in PICF IL‐1β presented a somehow more complex pattern: local concentrations were significantly higher at the end of the induction phase in DMT implants indicating a more intense inflammatory reaction. IL‐1β is a well‐characterized mediator and biomarker of bone loss in periodontal (Stashenko et al., 1991) and peri‐implant tissues (Faot et al., 2015). Its concentration in PICF correlates well with clinical measures of tissue inflammation, and the present findings corroborate the hypothesis that increased concentrations of IL‐1β in PICF are a key mechanism linking inflammation with peri‐implant bone loss and hence mucositis with peri‐implantitis.
The mucosal tunnel is a critical area for soft tissue health, and its depth is a complex parameter that is related to soft tissue height and thickness, depth of implant placement with reference to the crest of the neighbouring teeth and implant‐neck design. It is defined as the distance between the bottom of the sulcus (epithelial attachment to the implant) and the soft tissue (mucosal) margin and is delimited by the inner side of the soft tissue wall of the peri‐implant mucosa and the submucosal prosthetic components/crown. Oral biofilms extend into the mucosal tunnel along the surface of the implant prosthetic components and/or crown (Araujo & Lindhe, 2018). In specific clinical situations, it seems advantageous to obtain a DMT as it allows greater safety margin and greater distance to develop a natural looking shape to the tooth and thus ensure optimal aesthetic. Reports of implant placement in aesthetic areas have described peri‐implant probing depths as deep as 7 mm in clinically healthy and stable implants (Chappuis, Bornstein, Buser, & Belser, 2016). While there is some evidence that supragingival plaque control affects the composition of the subgingival microbiota in shallow pockets, it is generally maintained that the effectiveness of self‐performed oral hygiene below the gingival margin decreases as the distance from the margin increases (Waerhaug, 1981).
In this study, considerable variability was observed in terms of PICF biomarkers. This variability may be explained at least in part by the different resting volume of PICF at DMT and SMT (data not shown). The clear association observed between mGI and interleukin‐1β concentrations (Figure 4), however, lends added credibility to the differential time course that was observed. The data compare well with previous reports on peri‐implant mucosal inflammation (Faot et al., 2015) and are in general agreement with previous observations using the experimental gingivitis model (Offenbacher et al., 2010). Comparison of observed interleukin‐1β concentrations with those reported in previous studies is difficult due to: (a) methodological differences in the assays (high versus normal sensitivity ELISA); (b) methodological differences in sample collection (intra‐ and extra‐crevicular methods); (c) different approaches to determination of the concentration (timed sample versus volume adjusted sample); and (d) differences in the methods used for induction of peri‐implant mucositis (spontaneous biofilm accumulation versus stent). These differences explain, at least in part, the heterogeneity of baseline and peak concentrations observed across the different studies reporting on experimental and naturally occurring peri‐implant mucositis (Faot et al., 2015; Meyer et al., 2017; Salvi et al., 2012; Schincaglia et al., 2017). The pattern and the general time course of the changes observed in the SMT group, however, are consistent with those observed in previous studies.
While these data are limited to a single implant design with transmucosal platform, they are noteworthy as this type of design seems to have lower odds of peri‐implantitis compared to implants with bone level platforms (Derks et al., 2016a, 2016b).
The implications of these observations are far reaching. Maintenance of peri‐implant mucosal health requires self‐performed removal of biofilm deposits (Heitz‐Mayfield & Salvi, 2018). Peri‐implant mucositis seems to be highly prevalent (Derks & Tomasi, 2015; Derks et al., 2016a). Reversibility of peri‐implant mucositis with self‐performed oral hygiene measures or professional intervention is a key requirement for the prevention of peri‐implantitis (Jepsen et al., 2015). Systematic reviews summarizing the impact of self‐performed oral hygiene (Salvi & Ramseier, 2015) and professional interventions (Schwarz, Becker, & Renvert, 2015; Schwarz, Becker, & Sager, 2015) for peri‐implant mucositis show low rates of short‐term response. Clinical evidence from a 5‐year follow‐up study of peri‐implant mucositis shows that the problem resolves in only 30% of implants undergoing regular maintenance care and progresses to peri‐implantitis in almost 20% of cases (Costa et al., 2012). Another 5‐year follow‐up study shows lower incidence of peri‐implantitis during maintenance care that included removal of the prostheses at the six monthly recall appointments (Serino, Turri, & Lang, 2015). In this study, full reversibility of clinical measures of inflammation was observed only after a session of professional prophylaxis after temporary removal of the crowns.
The present data indicate that the depth of the mucosal tunnel may be a major modifier of the effectiveness of preventive measures of peri‐implantitis that require control of peri‐implant mucositis (Jepsen et al., 2015). The present data raise questions on treatment concepts that require deep submucosal placement of the implant platform to achieve better aesthetics, particularly in high‐risk subjects for higher, deregulated or non‐resolving chronic inflammation like those with a history of treated periodontitis. They also provide an opportunity to reflect on the most appropriate design of the cervical portion of the implant and its soft tissue interface. More investigations are warranted in this area to fully capture the parameters necessary for enabling effective resolution of peri‐implant mucositis and prevention of peri‐implantitis.
CONFLICT OF INTEREST
Authors report no conflict of interest related to this study.
5.
Clinical Relevance.
Scientific rationale for the study: Incomplete resolution of peri‐implant mucositis could be a key factor of developing peri‐implantitis. Implants with a deep mucosal tunnel (DMT) could be at risk of incomplete resolution of peri‐implant mucosal inflammation since oral hygiene practices do not extend deep below the soft tissue margin.
Principal findings: The depth of the mucosal tunnel modifies the resolution of experimental peri‐implant mucositis. Plaque accumulation and gingival inflammation change synchronously over time at implants with shallow mucosal tunnel. At implants with a DMT, there was a delayed and incomplete resolution of gingival inflammation in spite of effective plaque removal.
Practical implications: Depth of the mucosal tunnel (depth of implant placement with reference to the alveolar bone level of neighbouring teeth) seems to be an important parameter to consider for effective self‐performed oral hygiene and thus prevention and resolution of peri‐implant mucositis.
Chan D, Pelekos G, Ho D, Cortellini P, Tonetti MS. The depth of the implant mucosal tunnel modifies the development and resolution of experimental peri‐implant mucositis: A case–control study. J Clin Periodontol. 2019;46:248–255. 10.1111/jcpe.13066
Funding information
This study was supported by the European Research Group on Periodontology (ERGOPerio) and the University of Hong Kong Periodontal Research Fund.
REFERENCES
- Araujo, M. G. , & Lindhe, J. (2018). Peri‐implant health. Journal of Clinical Periodontology, 45(Suppl 20), S230–S236. 10.1111/jcpe.12952 [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Armitage, G. , Araujo, M. G. , Avila‐Ortiz, G. , Blanco, J. , Camargo, P. M. , … Zitzmann, N. (2018). Peri‐implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Clinical Periodontology, 45(Suppl 20), S286–S291. 10.1111/jcpe.12957 [DOI] [PubMed] [Google Scholar]
- Chappuis, V. , Bornstein, M. M. , Buser, D. , & Belser, U. (2016). Influence of implant neck design on facial bone crest dimensions in the esthetic zone analyzed by cone beam CT: A comparative study with a 5‐to‐9‐year follow‐up. Clinical Oral Implants Research, 27, 1055–1064. 10.1111/clr.12692 [DOI] [PubMed] [Google Scholar]
- Costa, F. O. , Takenaka‐Martinez, S. , Cota, L. O. , Ferreira, S. D. , Silva, G. L. , & Costa, J. E. (2012). Peri‐implant disease in subjects with and without preventive maintenance: A 5‐year follow‐up. Journal of Clinical Periodontology, 39, 173–181. 10.1111/j.1600-051X.2011.01819.x [DOI] [PubMed] [Google Scholar]
- Derks, J. , Schaller, D. , Hakansson, J. , Wennstrom, J. L. , Tomasi, C. , & Berglundh, T. (2016a). Effectiveness of implant therapy analyzed in a Swedish population: Prevalence of peri‐implantitis. Journal of Dental Research, 95, 43–49. 10.1177/0022034515608832 [DOI] [PubMed] [Google Scholar]
- Derks, J. , Schaller, D. , Hakansson, J. , Wennstrom, J. L. , Tomasi, C. , & Berglundh, T. (2016b). Peri‐implantitis ‐ onset and pattern of progression. Journal of Clinical Periodontology, 43, 383–388. 10.1111/jcpe.12535 [DOI] [PubMed] [Google Scholar]
- Derks, J. , & Tomasi, C. (2015). Peri‐implant health and disease. A systematic review of current epidemiology. Journal of Clinical Periodontology, 42(Suppl 16), S158–S171. 10.1111/jcpe.12334 [DOI] [PubMed] [Google Scholar]
- Faot, F. , Nascimento, G. G. , Bielemann, A. M. , Campao, T. D. , Leite, F. R. , & Quirynen, M. (2015). Can peri‐implant crevicular fluid assist in the diagnosis of peri‐implantitis? A systematic review and meta‐analysis. Journal of Periodontology, 86, 631–645. 10.1902/jop.2015.140603 [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. A. , & Salvi, G. E. (2018). Peri‐implant mucositis. Journal of Clinical Periodontology, 45(Suppl 20), S237–S245. 10.1111/jcpe.12953 [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. , Salvi, G. E. , Botticelli, D. , Mombelli, A. , Faddy, M. , & Lang, N. P. (2011). Anti‐infective treatment of peri‐implant mucositis: A randomised controlled clinical trial. Clinical Oral Implants Research, 22, 237–241. 10.1111/j.1600-0501.2010.02078.x [DOI] [PubMed] [Google Scholar]
- Jepsen, S. , Berglundh, T. , Genco, R. , Aass, A. M. , Demirel, K. , Derks, J. , … Zitzmann, N. U. (2015). Primary prevention of peri‐implantitis: Managing peri‐implant mucositis. Journal of Clinical Periodontology, 42(Suppl 16), S152–S157. 10.1111/jcpe.12369 [DOI] [PubMed] [Google Scholar]
- Katafuchi, M. , Weinstein, B. F. , Leroux, B. G. , Chen, Y. W. , & Daubert, D. M. (2018). Restoration contour is a risk indicator for peri‐implantitis: A cross‐sectional radiographic analysis. Journal of Clinical Periodontology, 45, 225–232. 10.1111/jcpe.12829 [DOI] [PubMed] [Google Scholar]
- Loe, H. , Theilade, E. , & Jensen, S. B. (1965). Experimental gingivitis in man. Journal of Periodontology, 36, 177–187. 10.1902/jop.1965.36.3.177 [DOI] [PubMed] [Google Scholar]
- Meyer, S. , Giannopoulou, C. , Courvoisier, D. , Schimmel, M. , Muller, F. , & Mombelli, A. (2017). Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses. Clinical Oral Implants Research, 28, 1005–1012. 10.1111/clr.12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombelli, A. , van Oosten, M. A. , Schurch Jr, E. , & Land, N. P. (1987). The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiology and Immunology, 2, 145–151. 10.1111/j.1399-302X.1987.tb00298.x [DOI] [PubMed] [Google Scholar]
- Offenbacher, S. , Barros, S. , Mendoza, L. , Mauriello, S. , Preisser, J. , Moss, K. , … Aspiras, M. (2010). Changes in gingival crevicular fluid inflammatory mediator levels during the induction and resolution of experimental gingivitis in humans. Journal of Clinical Periodontology, 37, 324–333. 10.1111/j.1600-051X.2010.01543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony, A. , Macneill, S. R. , & Cobb, C. M. (2000). Design features that may influence bacterial plaque retention: A retrospective analysis of failed implants. Quintessence International, 31, 249–256. [PubMed] [Google Scholar]
- Pontoriero, R. , Tonelli, M. P. , Carnevale, G. , Mombelli, A. , Nyman, S. R. , & Lang, N. P. (1994). Experimentally induced peri‐implant mucositis. A clinical study in humans. Clinical Oral Implants Research, 5, 254–259. 10.1034/j.1600-0501.1994.050409.x [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , Aglietta, M. , Eick, S. , Sculean, A. , Lang, N. P. , & Ramseier, C. A. (2012). Reversibility of experimental peri‐implant mucositis compared with experimental gingivitis in humans. Clinical Oral Implants Research, 23, 182–190. 10.1111/j.1600-0501.2011.02220.x [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , & Ramseier, C. A. (2015). Efficacy of patient‐administered mechanical and/or chemical plaque control protocols in the management of peri‐implant mucositis. A systematic review. Journal of Clinical Periodontology, 42(Suppl 16), S187–S201. 10.1111/jcpe.12321 [DOI] [PubMed] [Google Scholar]
- Schincaglia, G. P. , Hong, B. Y. , Rosania, A. , Barasz, J. , Thompson, A. , Sobue, T. , … Diaz, P. I. (2017). Clinical, immune, and microbiome traits of gingivitis and peri‐implant mucositis. Journal of Dental Research, 96, 47–55. 10.1177/0022034516668847 [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Becker, K. , & Renvert, S. (2015). Efficacy of air polishing for the non‐surgical treatment of peri‐implant diseases: A systematic review. Journal of Clinical Periodontology, 42, 951–959. 10.1111/jcpe.12454 [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Becker, K. , & Sager, M. (2015). Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri‐implant mucositis. A systematic review and meta‐analysis. Journal of Clinical Periodontology, 42(Suppl 16), S202–S213. 10.1111/jcpe.12349 [DOI] [PubMed] [Google Scholar]
- Serino, G. , & Strom, C. (2009). Peri‐implantitis in partially edentulous patients: Association with inadequate plaque control. Clinical Oral Implants Research, 20, 169–174. 10.1111/j.1600-0501.2008.01627.x [DOI] [PubMed] [Google Scholar]
- Serino, G. , Turri, A. , & Lang, N. P. (2015). Maintenance therapy in patients following the surgical treatment of peri‐implantitis: A 5‐year follow‐up study. Clinical Oral Implants Research, 26, 950–956. 10.1111/clr.12418 [DOI] [PubMed] [Google Scholar]
- Stashenko, P. , Fujiyoshi, P. , Obernesser, M. S. , Prostak, L. , Haffajee, A. D. , & Socransky, S. S. (1991). Levels of interleukin 1 beta in tissue from sites of active periodontal disease. Journal of Clinical Periodontology, 18, 548–554. 10.1111/j.1600-051X.1991.tb00088.x [DOI] [PubMed] [Google Scholar]
- Tonetti, M. , Cugini, M. A. , & Goodson, J. M. (1990). Zero‐order delivery with periodontal placement of tetracycline‐loaded ethylene vinyl acetate fibers. Journal of Periodontal, 25(4), 243–249. [DOI] [PubMed] [Google Scholar]
- Waerhaug, J. (1981). Effect of toothbrushing on subgingival plaque formation. Journal of Periodontology, 52, 30–34. 10.1902/jop.1981.52.1.30 [DOI] [PubMed] [Google Scholar]
- Zitzmann, N. U. , Berglundh, T. , Marinello, C. P. , & Lindhe, J. (2001). Experimental peri‐implant mucositis in man. Journal of Clinical Periodontology, 28, 517–523. 10.1034/j.1600-051x.2001.028006517.x [DOI] [PubMed] [Google Scholar]


