Figure 1.
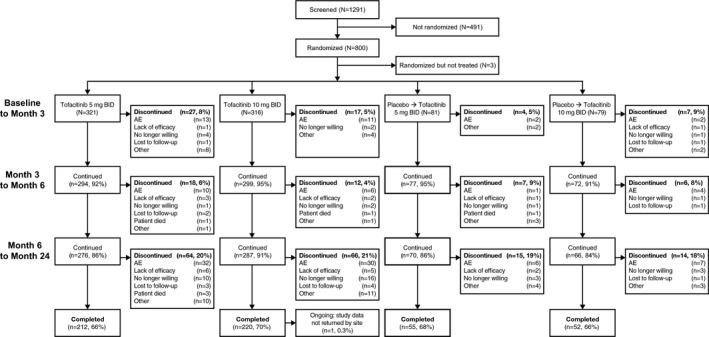
Disposition of the patients from baseline to month 24. Patients were randomized to receive tofacitinib 5 mg twice daily (BID), tofacitinib 10 mg twice daily, or placebo. All patients received background methotrexate, including those in the placebo groups. For patients in the placebo groups, treatment was switched in a blinded manner to tofacitinib 5 mg twice daily or tofacitinib 10 mg twice daily at either 3 months (for nonresponders) or 6 months (for all remaining patients in the placebo groups). Patients with <20% improvement in swollen and tender joint counts were considered nonresponders. At month 3, a <20% improvement in swollen and tender joint counts from baseline was found in 84 (26%) of the patients receiving tofacitinib 5 mg twice daily, 56 (18%) of the patients receiving tofacitinib 10 mg twice daily, 42 (52%) of the patients receiving placebo who switched to tofacitinib 5 mg twice daily, and 37 (47%) of the patients receiving placebo who switched to to tofacitinib 10 mg twice daily. Only deaths that occurred during study treatment are included. See Table 3 for details on deaths that occurred after the last dose of study drug. AE = adverse event.
