Table 3.
Cyclopropanation by 5 a–e from 1 a–e (left) or through decarbenation of 4 a (right).[a]
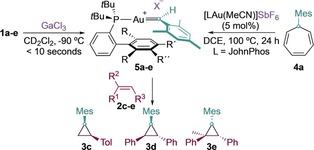
| Entry | Precursor | Yield [%][b] | ||
|---|---|---|---|---|
| 3 c | 3 d | 3 e | ||
| 1 | 1 a | 99 | 80 | 99 (2.3:1) |
| 2 | 1 b | 87 | 90 | 99 (3:1) |
| 3 | 1 c | 99 | 99 | 99 (1:1.2) |
| 4 | 1 d | 99 (1:1.5)31 | 99 | 72 (1:2.1) |
| 5 | 1 e | 99 | 90 | 99 (3.5:1) |
| 6 | 4 a | 32 | 38 | 17 (1:7.5) |
[a] Yields determined by 1H NMR spectroscopy using Ph2CH2 as the internal standard. [b] Diastereomeric ratio given within parenthesis. DCE=1,2‐dichloroethane.
