Figure 8.
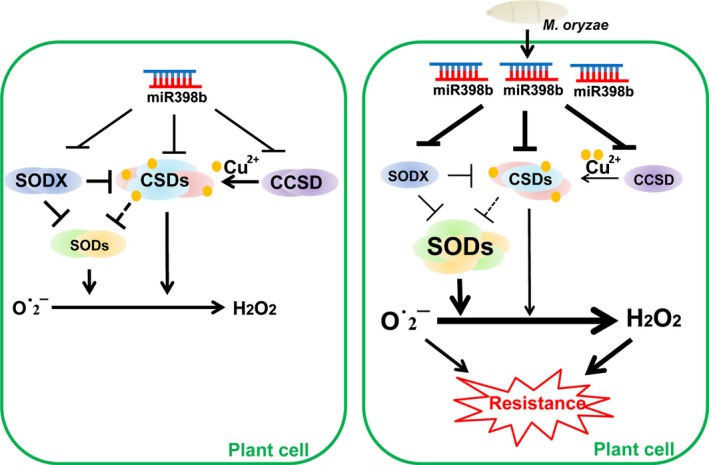
A model of miRNA398b functions in plant immunity to Magnaporthe oryzae. Without pathogen infection, miR398b‐regulated Cu/Zn‐Superoxidase Dismutase (CSD)1, CSD2, Superoxidase Dismutase (SOD)X and Copper Chaperone for Superoxide Dismutase (CCSD) maintain a basal CSD and total SOD enzyme activity level. CCSD positively contributes to CSD activity by transferring coppers to CSD protein. By contrast, SODX negatively regulates CSD and SOD activity by unknown mechanisms. A compensatory regulation mechanism exists between CSD and other SODs to maintain the hydrogen peroxide (H2O2) concentrations for normal metabolism in cells. Upon M. oryzae infection, the elevated miR398b accumulation represses the expression of CSDs/SODX/CCSD. On the one hand, the decreased CSDs and CCSD leads to lower CSD enzyme activity, which in turn results in higher expression and activity of other SODs by the unknown compensatory regulation mechanism. On the other, the decreased SODX compromises the repression on CSDs and SODs, and results in higher total SOD activity. As a result, the enhanced total SOD enzyme activity generates more H2O2 synthesis and results in enhanced resistance. Arrows indicate positive regulation and blunt‐ended bars indicate inhibition. The dotted lines indicate unidentified regulation between CSDs and SODs.
