Figure 2.
Overview of Substrate-Bound ClpB-DWB-K476C
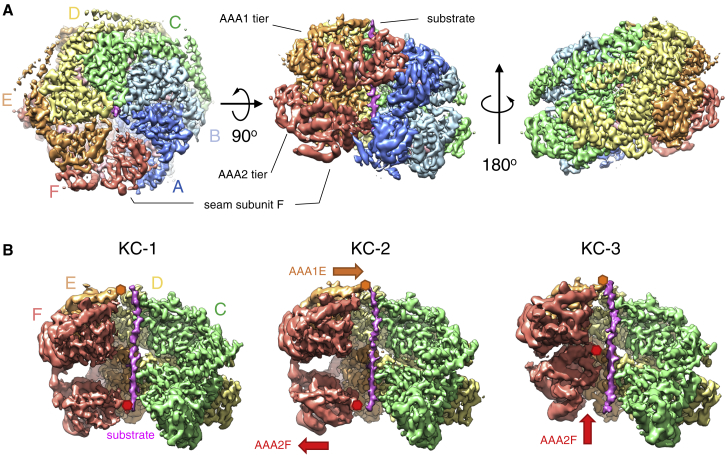
(A) Left, top view, and middle and right, side views of the cryo-EM density map of the most populated conformation of casein-bound ClpB-DWB-K476C (KC-2). The six protomers form a closed ring with a helical arrangement of two stacked AAA tiers and a seam between subunits A and F. The flexible N-terminal domains, located above the AAA1 tier, are not visible at high contour level. M-domains are partly visible for protomers C–E.
(B) Views of the cryo-EM maps of the three states of substrate-bound ClpB-DWB-K476C. Densities of protomers A and B are removed to show conformational changes in protomers AAA1E and AAA2F, highlighted by orange and red arrows, respectively. Orange and red hexagons show the position of moving AAA1E and AAA2F pore loops.