Figure 4.
Activation and Inactivation of Subunits in the AAA2 Ring Are Directly Coupled
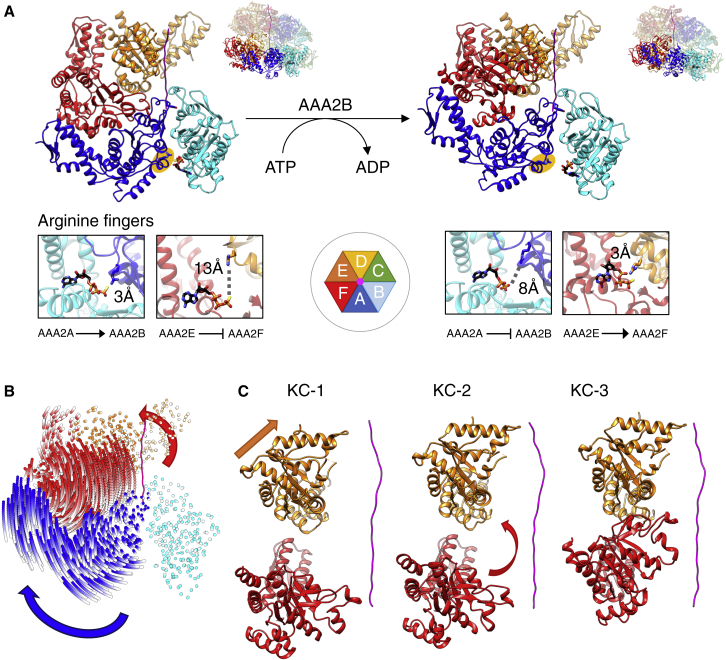
(A) Views of the AAA2 domains of protomers A, B, E, and F for states KC-2 and KC-3 are shown. The small lobe of AAA2B is omitted for clarity. In state KC-2 (left), the arginine finger of AAA2A (highlighted by yellow oval) contacts the γ-phosphate of ATP bound at neighboring AAA2B. In the post-hydrolysis state KC-3 (right), detachment of this arginine finger allows rotation of AAA2A by 14° to move away from AAA2B while remaining bound to the substrate. This rotation is transmitted to AAA2F, causing its repositioning to the top of the spiral track of AAA2 pore loops and its activation by receiving an arginine finger from AAA2E.
(B) The track of Cα atoms when morphing from KC-2 to KC-3 illustrates the amplitude of movements of AAA2A and AAA2F at the seam of the AAA2 ring. Blue and red arrows highlight the rotations of AAA2A and AAA2F subunits, respectively.
(C) Activation of AAA1E is a prerequisite for AAA2F rotation. Views of AAA1E, AAA2F, and casein substrate in states KC-1, KC-2, and KC-3 are shown.