Figure 4.
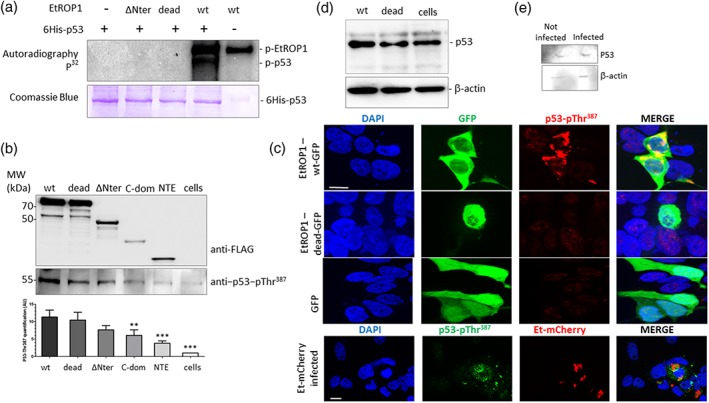
EtROP1 phosphorylates p53 on residue Thr387. (a) SDS‐PAGE autoradiography of a kinase assay (top panel) performed on EtROP1‐FLAG recombinant proteins (WT, dead and ∆Nter forms) and a 6His‐tagged p53 recombinant protein (detected by SDS‐PAGE followed by Coomassie blue staining, bottom panel). (b) Western blot analysis showing p53 phosphorylated Thr387 residue (p53‐pThr387) co‐purification with EtROP1‐FLAG wt, dead, ∆Nter, C‐dom and NTE forms in a pull‐down assay using anti‐FLAG affinity‐chromatography after constructs transfection in HEK293T cells. EtROP1‐FLAG constructs (wt, dead, ∆Nter, C‐dom and NTE; top panel) and p53‐pThr387 (bottom panel) were detected using an anti‐FLAG or an anti‐p53‐pThr387 antibody, respectively. Western blot signal were quantified by densitometry and ImageJ. (c) Upper panel: fluorescence microscopy analysis showing GFP (green) and phosphorylated p53 (anti‐p53‐pThr387 antibody, red) detection in HEK293T cells transfected with EtROP1‐GFP expression plasmids (wt and dead forms) or the control plasmid pcDNA‐GFP. Lower panel: phosphorylated p53 (anti‐p53‐pThr387 antibody, green) detected in HEK293T cells infected with E. tenella expressing mcherry (red). Bar represents 10 μm. (d) Western blot analysis showing p53 stabilisation in HEK293T cells transfected with EtROP1‐GFP expression plasmids (wt and dead forms) or the control plasmid pcDNA‐GFP. Two days posttransfection, GFP positive cells (transfected cells) were flow cytometry sorted before immunoblot analysis using an anti‐p53 antibody. An anti‐β‐actin antibody was used as an internal standard of immunoblot (IB). (e) Western blot analysis showing p53 stabilisation in epithelial caecal cells infected with E. tenella. Eighty‐four hours postinfection, mCherry positive caecal cells (infected cells) were flow cytometry sorted for subsequent total RNA purification. Analysis was run as in 4D legend
