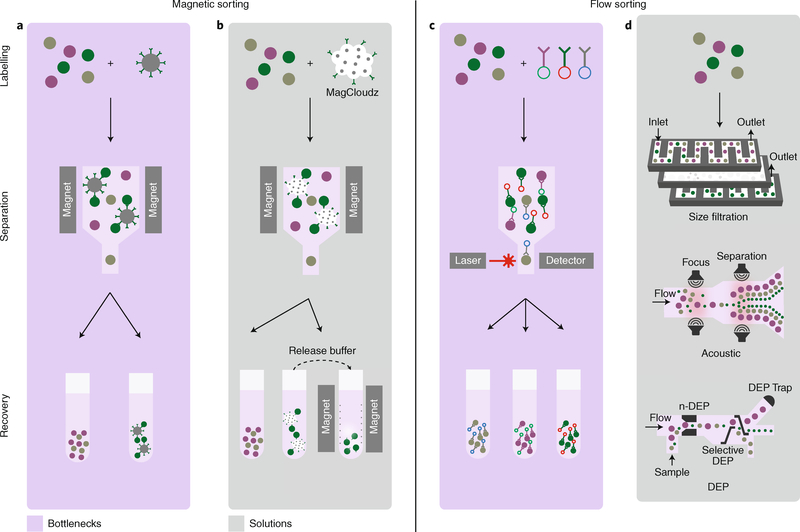
Fig. 3 |. Towards high-throughput label-free purification.
Cell-separation techniques, in which cells are first identified and labelled, and then separated and recovered, can currently be broken down into two main categories: magnetic sorting and flow sorting. a, Magnetic sorting uses magnetic beads coated with an antibody to separate cells from a mixed population (green, purple and grey). Current magnetic methods result in a positively selected population (green) that still has magnetic beads attached, which is undesirable (bottom right, recovered population). b, This issue has been circumvented by the MagCloudz QuadGel technology, which embeds magnetic particles into a hydrogel coated by antibodies, thereby effectively eliminating direct contact between the cells and the magnetic beads by using a release buffer that separates the magnetic particles from the hydrogel, which can then be recovered by magnets. c, Flow-cytometry sorting is a widely used method, based on fluorescently tagged antibodies (top right), that allows populations of cells to be selected according to antibody binding. The method is expensive and costly to set up in parallel; hence, it is typically used at low throughput. d, In microfluidic methods, which increase throughput for unlabelled cells, a mixed population of cells is passed through microchannels, where cell separation is driven by a sequence of events, such as size filtering, acoustic separation and dielectrophoresis (DEP) sorting. A DEP trap can be set to collect the desired cells. n-DEP, negative dielectrophoresis.