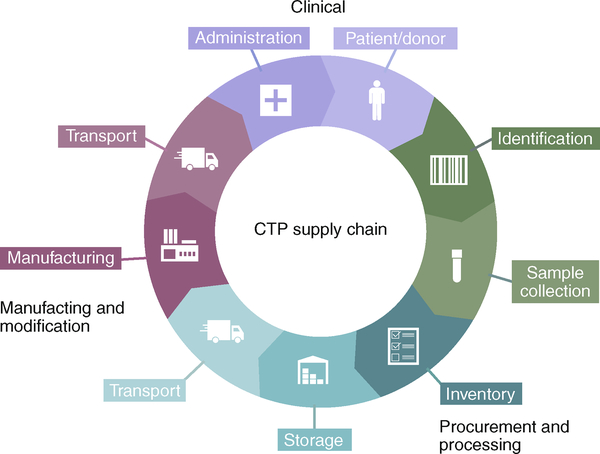
Fig. 7 |. Supply chain for CTPs.
The CTP supply chain is a complicated flow process comprising a series of dynamic components starting in a clinical environment, going through bioprocessing and then returning to the clinic. Initial seeding products are derived from patients or donors. These are screened for health and for safety (identification). Once cleared, sample collection begins. Proper inventory must then be made for tracking purposes. The cells are then put into storage (either short term or long term, depending on whether they are meant for banking or for immediate use). Transportation of the cells proceeds to the manufacturing facility, where purification, modification and/or expansion can take place. Once processing is complete, the product is moved onto the end location, for administration to the end patient. Supply-chain logistics are crucial to the overall success of CTPs.