Abstract
Background:
Laparoscopy is important for management of endometriosis patients with estimation of endometriosis fertility index (EFI) which can predict reproductive outcome.
Aims:
This study aims to evaluate clinical outcome in laparoscopically managed pelvic endometriosis and correlation of reproductive outcome with EFI.
Setting and Design:
Retrospective cohort study carried out in the Department of Obstetrics and Gynecology.
Materials and Methods:
Our study included 123 patients who had undergone laparoscopic management of endometriosis from January 2017 to March 2018. Case files were retrieved and meticulously analyzed. All patients were contacted and interviewed. Symptomatic relief and pregnancy in infertility patients were recorded. EFI was calculated.
Statistical Analysis:
Data analyses were carried out using statistical software STATA version 12.0. P < 0.05 was considered statistically significant.
Results and Conclusions:
A total of 123 cases were enrolled; the most common complaint was infertility 107 (86.99%); the mean age was 32.4 years. EFI was found to be (6 to 10) in 28(26.2%) patients, EFI of (4 to 5) in 49 (45.8%) and EFI of (0 to 3) in 30 (28.0%). Post surgery, dysmenorrhoea was relieved in 56 (65.88%) patients, menstrual irregularities were relieved in 45 (76.27 %) patients, dyspareunia in 32 (54.24%) and chronic pelvic pain in 24 (40.5%) patients. 8 (40%) patients with low EFI conceived, 20 (58.82%) with moderate, and 26 (96.29%) with high EFI conceived. EFI score showed statistically significant positive correlation with pregnancy outcome P = 0.001, higher the EFI score, better the reproductive outcome. Laparoscopic surgeries are important for managing patients of endometriosis. It provides significant symptomatic relief, and EFI estimation can be done, which is a good tool to predict reproductive outcome of infertility patients with endometriosis.
KEYWORDS: Endometriosis, endometriosis fertility index, infertility, laparoscopy, least function score, surgery
INTRODUCTION
Endometriosis is the presence of endometrial glands and stroma outside the endometrial cavity. It is a hormone-dependent chronic disease chiefly found in reproductive age group. It has varied clinical presentations, which range from asymptomatic to symptoms such as pelvic pain, dysmenorrhea, irregular menstruation, infertility, adnexal mass, dyspareunia, bladder, and bowel complaints. Its incidence is about 35%–50% in women of reproductive age group.[1] Around 30%–50% of women with endometriosis suffer from infertility.[2] Endometriosis causes infertility by several mechanisms such as distortion of pelvic anatomy,[3] altering function of peritoneum,[4] alteration of hormonal and cell-mediated functions,[5] endocrine and ovulatory dysfunction,[6] impaired implantation,[7,8] abnormalities in oocyte and embryo quality,[9] and impairment of uterotubal transport.[10] Laparoscopy is the gold standard diagnostic tool. Various laparoscopic surgeries for endometriosis include adhesiolysis, excision/ablation of endometriotic implants, cystectomy, cyst drainage, and oophorectomy. Various classification systems are in place to classify and stage endometriosis, the most widely used is the revised American Society of Reproductive Medicine (rASRM). This classification system is based on laparoscopic visualization of the endometriotic lesion, and it classifies endometriosis into minimal, mild, moderate, or severe. Even though this classification is well enough to classify the severity of endometriosis, it does not predict the outcome of treatment, especially reproductive outcome.[11] Hence, a new classification system has been developed as endometriosis fertility index (EFI) to predict clinical outcome in surgically documented endometriosis patients with infertility.[12]
Aims and objectives
To evaluate clinical outcome in laparoscopically managed pelvic endometriosis and correlation of reproductive outcome with EFI.
Type of study
Retrospective cohort study.
MATERIALS AND METHODS
This institute is a tertiary care institute with Department of Obstetrics and Gynaecology being a referral laparoscopy center for endometriosis and fertility-enhancing surgeries. Our study included 123 patients who had undergone laparoscopic management of endometriosis from January 2017 to March 2018. Case files of all these patients were retrieved and meticulously analyzed to record the personal particulars, presenting complaints, and details of surgery performed. All these patients were contacted and interviewed telephonically and through mails. Symptomatic relief and pregnancy in case of infertility patients were recorded. Since the duration of follow-up was short, pregnancy was considered as positive outcome with sonologically confirmed intrauterine live pregnancy. Other symptomatic outcomes were analyzed as per patient's perception of improvement in clinical symptoms as compared with symptomatology at the time of surgery. A Numerical Rating Scale where the respondent selects a whole number between 0 and 10 was used to scale their symptomatic relief from dysmenorrhea, dyspareunia, chronic pelvic pain, and menstrual complaints.
All patients who presented with the specific symptoms of endometriosis with or without associated infertility underwent pelvic imaging, with ultrasonography ± magnetic resonance imaging. Preoperative imaging findings were recorded in the case records. All patients underwent standard laparoscopic management for pelvic endometriosis. The surgeries included laparoscopic fulguration of endometriotic spots, adhesiolysis, endometriotic cystectomy, cyst drainage, or oophorectomy. Surgical findings were recorded in rASRM pro forma as depicted in Figure 1.
Figure 1.
Revised American Society of Reproductive Medicine classification of endometriosis surgical staging pro forma
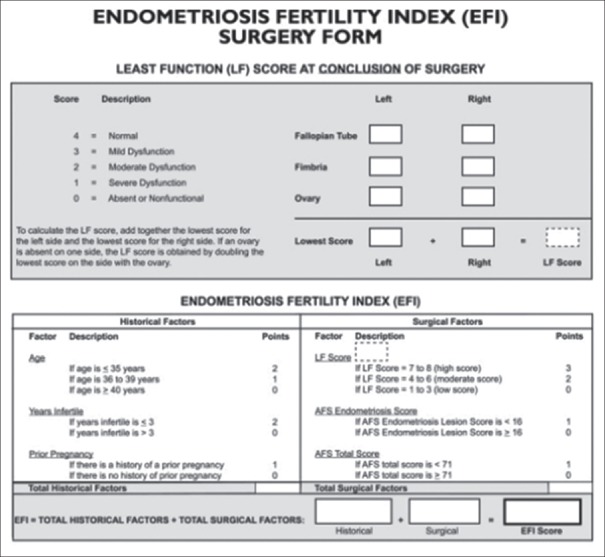
All infertility patients underwent intraoperative chromopertubation for checking tubal patency. At the conclusion of surgery, least function score (LFS) was calculated for each patient as a postoperative function of fallopian tubes, fimbriae, and ovaries. EFI was calculated as the sum of the historical factors (age, status of prior pregnancy, and years of infertility) and surgical factors (LFS, American Fertility Society [AFS] endometriosis lesion score, and AFS total score), as depicted in Figure 2.
Figure 2.
Endometriosis fertility index surgery form
Statistical analysis
Data analyses were carried out using statistical software STATA version 12.0 (StataCorp, 2011, Stata Statistical Software: Release 12 College Station, TX, StataCorp LP). For categorical variables, frequency and percent values were presented. Comparison of frequency data across categories was performed by Chi-square/Fisher's exact test as appropriate. Continuous variables were tested for normality assumption using Kolmogorov–Smirnov test. For variables that were found to be approximate to normal distribution, descriptive statistics such as mean and standard deviation were calculated. Comparison of mean values was carried out using Student's t-independent test. Odds ratio and 95% confidence limits were calculated wherever appropriate. For all statistical tests, a two-sided probability of P < 0.05 was considered as statistical significance.
RESULTS
A total of 123 patients who underwent laparoscopic surgeries for endometriosis from January 2017 to March 2018 were enrolled in the study. Their data were analyzed in detail, and symptomatic relief and reproductive outcome were followed for 6 months after surgery for endometriosis. Patients usually had overlapping symptoms, the most common complaint for which the patient sought treatment was infertility in 107 patients (86.99%), followed by dysmenorrhea in 85 patients (69.1%), chronic pelvic pain in 69 patients (56.09%), dyspareunia in 59 (47.96%), menstrual irregularities in 59 (47.9%), adnexal mass in 12 (9.75%), and bladder/bowel complaints in 5 patients (4.01%). Out of the total 107 infertility patients, 88 (82.24%) had primary infertility and 19 (17.75%) had secondary infertility. Duration of infertility was found to be 3 years in 15 (14.02%) patients and >3 years in 92 (85.98%) patients. 77 (62.60%) patients were of age 35 years, 36 (29.27%) between 36 and 39 years of age, and 10 (8.13%) aged 40 years. The mean age was 32.4 years. Twenty-nine patients were lost to follow-up of which 26 had infertility, and conception rate could be assessed in only 81 patients. Based on the peroperative findings, severity of endometriosis was graded and staged as per rASRM classification. Mild Stage I disease was found in 3 (2.43%) patients, minimal Stage II disease in 18 (14.6%), moderate Stage III disease in 89 (72.35%) patients, and severe Stage IV disease in 13 (10.56%) patients. LFS was documented for every infertility patient after surgery. Forty-six (43%) patients had low LFS of 1–3, 53 (49.5%) patients had moderate LFS of 4–6, and 8 (7.5%) patients had high LFS of 7–8. AFS endometriosis lesion score was found to be >16 in 62 (57.9%) patients and <16 in 45 (42.1%) patients. AFS total score was 71 in only 3 (2.8%) patients, and 104 (97.2%) had AFS total as <71. EFI was found to be 6–10 in 28 (26.2%) patients, EFI of 4–5 in 49 (45.8%) and EFI of 0–3 in 30 (28.0%) [Table 1]. Twenty-eight patients with EFI 6–10 were advised to try for spontaneous conception, 49 patients with EFI 4–5 advised ovulation induction (OVI) with or without intrauterine insemination (IUI) depending on husband's semen analysis, and 30 patients with EFI 0–3 given an option between OVI ± IUI and in vitro fertilization (IVF) depending on economic status of the patient.
Table 1.
Characteristics of patients
| Characteristics | n (%) |
|---|---|
| Age at the time of surgery (years) | |
| <35 | 77 (62.60) |
| 36-39 | 36 (29.27) |
| >40 | 10 (8.13) |
| Duration of infertility | |
| <3 | 15 (14.02) |
| >3 | 92 (85.98) |
| Type of infertility | |
| Primary | 88 (82.24) |
| Secondary | 19 (17.75) |
| Least function score | |
| High score (7-8) | 8 (7.5) |
| Moderate score (4-6) | 53 (49.5) |
| Low score (1-3) | 46 (43) |
| AFS endometriosis lesion score | |
| <16 | 45 (42.1) |
| >16 | 62 (57.9) |
| AFS total score | |
| <71 | 104 (97.2) |
| >71 | 3 (2.8) |
AFS=American Fertility Society
The various surgeries performed for endometriosis ranged from adhesiolysis, cystectomy, and fulguration of endometriotic spots in 56 (45.5%) patients, adhesiolysis and cystectomy in 43 (35%) patients, fulguration of endometriotic spots in 11 (8.9%) patients, adhesiolysis and endometriotic cyst drainage in 10 (8.1%) cases, and adhesiolysis with oophorectomy in 3 (2.4%) patients. Most of the surgeries were performed within 1–2 h in 88 (71.5%), <1 h in 23 (18.7%), 2–3 h in 9 (7.3%) patients, and >3 h in 3 (2.4%) patients. Most of the patients were discharged the same day of surgery, i.e., 108 (87.80%). Two patients had bladder injury which was detected intraoperatively and repaired, three patients had bowel injuries which were also detected during surgery and repaired, and 5 patients needed blood transfusion in the postoperative period.
Dysmenorrhea was relieved in 56 (65.88%) patients, menstrual irregularities were relieved in 45 (76.27%) patients, dyspareunia in 32 (54.24%), and chronic pelvic pain in 24 (40.5%) patients. Among the 81 infertility patients who were followed, conception rate was 66.7%. A total of 27 (33.3%) patients did not conceive, 19 (23.5%) patients conceived spontaneously, 24 (29.6%) patients conceived on OVI with or without IUI, and 11 (13.6%) conceived by IVF. Among the various EFI groups 8 (40%) patients with low EFI (0-3) conceived (all by IVF), 20 (58.82%) patients with moderate EFI (4-5) conceived (17 with OVI ± IUI, 3 with IVF) and 26 (96.29%) with high EFI (6-10) conceived (19 spontaneously and 7 with OVI ±I UI). Chi-square test was used to assess the association of EFI with various factors and conception rate [Table 2]. EFI showed significant relationship with years of infertility P nfe. 014, lower EFI score was seen in patients with duration of infertility of >3 years. Significant association was seen of EFI with previous history of pregnancy (primary/secondary infertility) P fe. 001, higher EFI score in patients with previous history of pregnancy. EFI also had significant association with age of the patient P at0.001, lower EFI score seen with advancing age of the patient. EFI showed significant association with stage of disease P ise. 001, more severe the disease, lower the EFI score. EFI had statistically significant association with LFS P cor. 001, lower LFS associated with lower EFI score. EFI also had statistically significant association with AFS endometriosis score P cor. 001, more the AFS endometriosis score, lesser the EFI [Table 2]. Age showed significant association with conception rate P ate. 03, less conception rate seen with increased age. EFI score showed statistically significant positive correlation with pregnancy outcome P = 0.001, higher the EFI score, better the reproductive outcome.
Table 2.
Endometriosis fertility index score and conception rate
| EFI score | Conceived | Spontaneously | OVI±IUI | IVF | Not conceived | Total |
|---|---|---|---|---|---|---|
| Low (0-3) | 8 (40) | 0 | 0 | 8 (100) | 12 (60) | 20 |
| Moderate (4-5) | 20 (58.82) | 0 | 17 (85) | 3 (15) | 14 (41.18) | 34 |
| High (6-10) | 26 (96.29) | 19 (73.08) | 7 (26.92) | 0 | 1 (3.71) | 27 |
| Total | 54 | 19 | 24 | 11 | 27 | 81 |
Comparison of Odd’s Ratio: Low EFI vs Moderate EFI: OR 0.47 (95% CI: 0.13-1.65); P=0.181. Low EFI vs High EFI: OR 0.03 (95% CI: 0.00-0.24); P=0.001. Moderate EFI vs High EFI: OR 0.05 (95% CI: 0.0-0.43); P=0.001
DISCUSSION
Endometriosis is a disease of women in reproductive age group. The mean age of patients in our study was 32.4 years which is in accordance with 31.23 years reported by Alborzi et al.[13] In another study by Bellelis et al., the mean age at the time of diagnosis was reported to be 33.2 years.[14] Endometriosis presents with a wide range of symptoms, and often, the symptoms do not correlate with the severity of disease. In a 2017 summary of NICE guidance for diagnosis and management of endometriosis, the signs and symptoms of endometriosis included chronic pelvic pain, dysmenorrhea, infertility, painful bowel, and bladder complaints.[15] Vercellini et al. in a prospective study stated the presenting complaints in endometriosis as chronic pelvic pain in 31.2% patients, pelvic mass in 29.7%, and infertility in 29.6% patients.[16] In our study, 86.99% patients presented with infertility. There was overlapping of symptoms; patients also had associated symptoms such as dysmenorrhea in 69.1%, chronic pelvic pain in 56.09%, and dyspareunia in 47.96% patients. Alborzi et al[13] in their study of surgical outcomes in endometriosis surgery, reported an incidence of 53.68 % stage IV severe endometriosis, 28.9% moderate stage III disease, 9.05% minimal stage I and 8.37% mild stage II disease. In our study, there was incidence of 72.35% moderate Stage III disease, 10.56% severe Stage IV, 2.43% minimal stage I disease, and 14.6% mild Stage II disease.
Laparoscopic surgical therapy is the gold standard in the treatment of symptomatic pelvic endometriosis. Type of surgery performed in the laparoscopic management of endometriosis depends on patient's age, symptoms, extent and severity of disease, type and location of lesion, fertility concern, etc. Sutton et al.,[17] Abbott et al.,[18] Jarrell et al.,[19] and Vercellini et al.[20] in their studies on the effectiveness of laparoscopic surgeries for symptomatic Stage I-IV endometriosis, pain relief was reported in 30%–40% patients during short follow-up. In our study, dysmenorrhea was relieved in 56 (65.88%) patients, dyspareunia in 32 (54.24%) patients, and chronic pelvic pain in 24 (40.5%) patients.
Adamson and Pasta[12] developed a new staging system and a clinical tool to predict reproductive outcome in surgically treated endometriosis patients with infertility. LFS was calculated to ascertain EFI intraoperatively after surgical intervention was done that denoted the function of tube, fimbria, and ovary on both sides. They concluded that the EFI is a simple, robust, and validated clinical tool that predicts pregnancy rates after endometriosis surgical staging. In a retrospective study by Zhang et al.[21] on prediction of EFI in patients with endometriosis-associated infertility after laparoscopic treatment, the duration of infertility <3 years was seen in 72.38% patients and >3 years in 27.62% patients. About 48.95% patients had primary infertility and 51.05% patients presented with secondary infertility. In the same study, 90.1% patients were in the age group <35 years, 7.93% in 36–39 years, and 1.91% in >40 years of age. In our study, 62.60% patients were of age <35 years, 29.27% between 36 and 39 years of age, and 8.13% aged >40 years. Furthermore, duration of infertility was found to be <3 years in 14.02% patients and >3 years in 85.98% patients. Of 107 infertility patients, 82.24% had primary infertility and 17.75% had secondary infertility which were different from the study under reference as our study was conducted in a tertiary referral center of a developing nation which may be lacking a proper referral system.
Zhang et al.[21] in their study found that 12.58% patients were with low LFS score, 52.32% with moderate LFS, and 35.10% with high LFS score. AFS endometriosis lesion score was found to be <16 in 81.49% and >16 in 18.51%. AFS total score was found to be <71 in 91.89% patients and >71 in 8.11%. The calculated EFI scores were 10 in 128 patients, 8–9 in 501 patients, 6–7 in 338 patients, 4–5 in 111 patients, and 2–3 in 19 patients. In their study, 46.03% patients conceived naturally. The cumulative pregnancy incidence among the EFI scores of 10, 7–9, 4–6, and 2–3 was statistically significant (P = 0.001) and increased with increasing EFI score. In our study, 43% patients had low LFS of 1–3, 49.5% patients had moderate LFS of 4–6, and 7.5% patients had high LFS score of 7–8. AFS endometriosis lesion was found to be >16 in 57.9% patients and ≤16 in 42.1% patients. AFS total was w71 in only 2.8% patients of infertility and 97.2% had AFS total as <71. EFI score was 6–10 in 28 (26.2%) patients, 4–5 in 49 (45.8%), and 0–3 in 30 (28.0%). In our study, overall conception rate was 66.7%. EFI score showed statistically significant positive correlation with pregnancy outcome P = 0.001, higher the EFI score, better the reproductive outcome.
CONCLUSIONS
Laparoscopic surgeries are important for managing patients of endometriosis. It provides significant symptomatic relief, and EFI estimation can be done, which is a good tool to predict reproductive outcome of infertility patients with endometriosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784–96. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- 3.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–98. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pizzo A, Salmeri FM, Ardita FV, Sofo V, Tripepi M, Marsico S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Invest. 2002;54:82–7. doi: 10.1159/000067717. [DOI] [PubMed] [Google Scholar]
- 5.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- 6.Cunha-Filho JS, Gross JL, Bastos de Souza CA, Lemos NA, Giugliani C, Freitas F, et al. Physiopathological aspects of corpus luteum defect in infertile patients with mild/minimal endometriosis. J Assist Reprod Genet. 2003;20:117–21. doi: 10.1023/A:1022625106489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 8.Cakmak H, Taylor HS. Molecular mechanisms of treatment resistance in endometriosis: The role of progesterone-hox gene interactions. Semin Reprod Med. 2010;28:69–74. doi: 10.1055/s-0029-1242996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrido N, Navarro J, García-Velasco J, Remoh J, Pellice A, Simón C. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update. 2002;8:95–103. doi: 10.1093/humupd/8.1.95. [DOI] [PubMed] [Google Scholar]
- 10.Kissler S, Hamscho N, Zangos S, Gätje R, Müller A, Rody A, et al. Diminished pregnancy rates in endometriosis due to impaired uterotubal transport assessed by hysterosalpingoscintigraphy. BJOG. 2005;112:1391–6. doi: 10.1111/j.1471-0528.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 11.Revised American Fertility Society classification of endometriosis: 1985. Fertil Steril. 1985;43:351–2. doi: 10.1016/s0015-0282(16)48430-x. [DOI] [PubMed] [Google Scholar]
- 12.Adamson GD, Pasta DJ. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil Steril. 2010;94:1609–15. doi: 10.1016/j.fertnstert.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Alborzi S, Hosseini-Nohadani A, Poordast T, Shomali Z. Surgical outcomes of laparoscopic endometriosis surgery: A 6 year experience. Curr Med Res Opin. 2017;33:2229–34. doi: 10.1080/03007995.2017.1362377. [DOI] [PubMed] [Google Scholar]
- 14.Bellelis P, Dias JA, Jr, Podgaec S, Gonzales M, Baracat EC, Abrão MS, et al. Epidemiological and clinical aspects of pelvic endometriosis – A case series. Rev Assoc Med Bras (1992) 2010;56:467–71. doi: 10.1590/s0104-42302010000400022. [DOI] [PubMed] [Google Scholar]
- 15.Kuznetsov L, Dworzynski K, Davies M, Overton C, Guideline Committee. Diagnosis and management of endometriosis: Summary of NICE guidance. BMJ. 2017;358:j3935. doi: 10.1136/bmj.j3935. [DOI] [PubMed] [Google Scholar]
- 16.Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: A multivariate analysis of over 1000 patients. Hum Reprod. 2007;22:266–71. doi: 10.1093/humrep/del339. [DOI] [PubMed] [Google Scholar]
- 17.Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696–700. doi: 10.1016/s0015-0282(16)56990-8. [DOI] [PubMed] [Google Scholar]
- 18.Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R, et al. Laparoscopic excision of endometriosis: A randomized, placebo-controlled trial. Fertil Steril. 2004;82:878–84. doi: 10.1016/j.fertnstert.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 19.Jarrell J, Mohindra R, Ross S, Taenzer P, Brant R. Laparoscopy and reported pain among patients with endometriosis. J Obstet Gynaecol Can. 2005;27:477–85. doi: 10.1016/s1701-2163(16)30531-x. [DOI] [PubMed] [Google Scholar]
- 20.Vercellini P, Crosignani PG, Abbiati A, Somigliana E, Viganò P, Fedele L. The effect of surgery for symptomatic endometriosis: The other side of the story. Hum Reprod Update. 2009;15:177–88. doi: 10.1093/humupd/dmn062. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Liu D, Huang W, Wang Q, Feng X, Tan J. Prediction of endometriosis fertility index in patients with endometriosis-associated infertility after laparoscopic treatment. Reprod Biomed Online. 2018;37:53–9. doi: 10.1016/j.rbmo.2018.03.012. [DOI] [PubMed] [Google Scholar]