Abstract
Background:
Impairments in central and/or peripheral nervous systems are known to be associated with altered gait; however, the interplay between cognitive function, peripheral sensation, and orbital gait stability remains largely unclear. Elucidating these relationships is expected to provide a clearer understanding of potential fall-risk factors across various populations and targets for novel interventions. Many patients diagnosed with cancer are treated with chemotherapy agents known to be neurotoxic to the central and/or peripheral nervous systems that can contribute to movement deficiencies, making this population a novel model to investigate these relationships.
Research Question:
The purpose of this exploratory study was to investigate how central and peripheral nervous system impairments associate with orbital stability during single- and dual-task gait.
Methods:
Twenty cancer survivors were enrolled and separated into three groups: no prior chemotherapy exposure (CON, n=6), and prior treatment with chemotherapy and having no/mild chemotherapy-induced peripheral neuropathy (CIPN) symptoms (-CIPN, n=8) or moderate/severe CIPN symptoms (+CIPN, n=6). Testing included single- and dual-task (i.e., serial sevens) treadmill walking as well as a computerized test of executive function. Maximum Floquet multipliers were calculated to assess orbital stability during gait.
Results:
Worse executive function was associated with decreased orbital stability during the dual-task condition in the +CIPN group (Spearman’s ρ = 0.94, P = 0.017). Additionally, decreased orbital stability during dual-task gait was observed for the -CIPN group compared to the CON group (ES = 1.96, P = 0.019).
Significance:
Executive dysfunction was associated with decreased gait stability during challenging dual-task gait in survivors with sensory symptoms of CIPN. The association between combined central and peripheral nervous system impairments and decreased gait stability in cancer survivors provides a novel demonstration of potential compensatory strategies that accompany deficiencies in these functions. Future work is needed to confirm these relationships and whether they hold in other populations.
Introduction
Impairments in central and/or peripheral nervous systems are known to be associated with altered gait; however, the interplay between cognitive function, peripheral sensation, and gait stability remains largely unclear. With respect to orbital stability, existing literature regarding these relationships is scarce and limited to studies of the isolated effects of peripheral or central nervous system interference, leaving the potential interaction between these factors unknown. Delineating isolated versus combined effects of peripheral and central nervous system impairments on orbital gait stability provides an opportunity to better understand potential compensatory strategies that occur during gait in the presence of various impairments.
Cancer survivors represent a novel model to investigate these relationships because common chemotherapy treatments can be neurotoxic to the peripheral and/or central nervous systems and these individuals have an increased risk of falling [1,2]. Chemotherapy-induced peripheral neuropathy (CIPN) is a leading dose-limiting side effect of commonly used chemotherapy agents that impair peripheral sensation, such as taxanes and oxaliplatin [3]. Existing research suggests that taxane-based chemotherapy [4] and CIPN [2,5–7] are associated with altered postural control, gait, and falls.
In addition to peripheral sensory impairments, chemotherapy-associated cognitive impairments may interfere with survivors’ movement quality. Deficits in cognitive domains including processing speed, executive function, working memory, and visuospatial abilities have been reported in 10–70% of cancer survivors, with a subgroup (17–34%) of cancer survivors having long-term cognitive impairments [8,9]. Impairments in executive function are associated with increased gait variability and decreased average walking speed, stride length, and stride time, particularly in dual-task scenarios (i.e., attending to two concurrent tasks) [10,11]. These findings support the need to understand the potential role of cognitive impairments in gait instability.
Measures of gait stability can provide unique information for characterizing gait impairments. Nonlinear measures have been implemented in gait analyses to provide more direct measures of gait stability (i.e., response of the system to a perturbation) [12], with potential relevance to falls [13,14]. To our knowledge, gait stability has not been investigated in cancer survivors, but may yield new insight into gait impairments demonstrated by individuals with peripheral and/or central nervous system impairments.
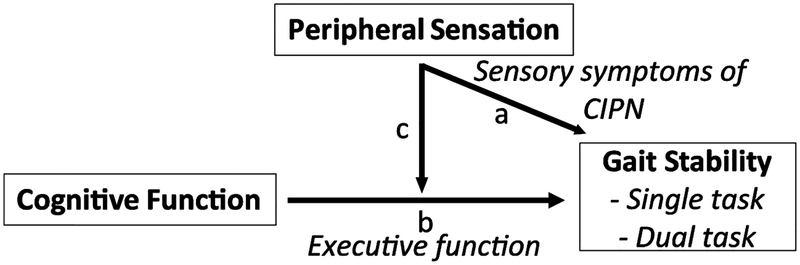
The purpose of this exploratory study was to investigate gait impairments associated with central and/or peripheral nervous system impairments (Figure 1). The hypotheses were (1) impaired peripheral sensation related to CIPN would be associated with decreased orbital stability during gait, and (2) participants with CIPN and cognitive impairments would demonstrate worsened gait impairments during dual-task walking.
Figure 1.
Schematic representation of the relationships of interest. a) How does altered peripheral sensation (i.e., sensory symptoms of CIPN) associate with gait stability? b) Are estimates of cognitive function (executive function) correlated with gait stability? c) How does altered peripheral sensation influence the potential relationships between cognitive function and gait stability?
Materials and Methods
Participants
Breast (stages I-III) and colorectal (stages I-IV) cancer patients were recruited from the following three groups: (1) diagnosed with cancer, but not requiring chemotherapy (CON), (2) diagnosed with cancer, received taxane or oxaliplatin chemotherapy, and no to mild symptoms of CIPN (-CIPN), and (3) diagnosed with cancer, received taxane or oxaliplatin chemotherapy, and developed moderate to severe symptoms of CIPN (+CIPN). These groups were chosen to attempt to delineate the effects associated with CIPN compared to other cancer diagnosis and/or treatment-related effects [7]. Individuals unable to stand or walk without assistance or having a pre-existing diagnosis of neuropathy of any kind were excluded from the study. Individuals with a prior lower extremity joint replacement surgery were also excluded.
Protocol
-CIPN and +CIPN participants completed a testing session within approximately 6 weeks of completing chemotherapy (mean: 3.8 weeks; range: 1.1 – 6.3 weeks). CON participants were all at least 6 weeks post-surgery. During this visit, patients completed single- and dual-task treadmill walking, a computerized executive function test, and questionnaires of patient-reported outcomes.
Treadmill walking consisted of an acclimation period and two 5-minute recorded trials. Participants walked on a split belt instrumented treadmill (Bertec Corp.; Columbus, OH). Self-selected walking speed was identified during the acclimation period using a modified procedure from Jordan et al. [15], where our initial speed was set to 0.5 m/s and 0.05 m/s increments/decrements were then made. Two participants selected speeds slower than the initial 0.5 m/s speed (one-CIPN participant and one +CIPN participant). After identifying the self-selected speed, participants continued to walk at that speed until they were comfortable with the experimental setup.
Participants then took a 2-minute break before completing the first of two 5-minute walking trials that were performed at the same pre-determined self-selected walking speed (Table 1). The trials consisted of one single-task (ST) and one dual-task (DT) trial. The trial order was randomized between participants. A 2-minute break was given between tasks. The DT condition consisted of a serial 7’s subtraction where participants counted down by 7’s from 1000. Participants were instructed to do their best to maintain focus on the serial 7’s task throughout the duration of the trial. Accuracy of the serial 7’s task was not recorded, and one participant refused to count by 7’s and instead counted down by 4’s. The ST trial consisted of walking with minimized distractions as participants were instructed to maintain their gaze straight ahead.
Table 1.
Participant demographic, treatment, and symptom characteristics.
| General Characteristics, mean ± SD | CON (n=6) |
−CIPN (n=8) |
+CIPN (n=6) |
|---|---|---|---|
| Age (yr) | 59.3 ± 9.6 | 55.9 ± 9.0 | 50.0 ± 15.0 |
| Male/Female | 0/6 | 1/7 | 2/4 |
| Mass (kg) | 78.0 ± 14.1 | 77.1 ± 11.1 | 91.6 ± 21.6 |
| Height (m) | 1.62 ± 0.05 | 1.68 ± 0.04 | 1.71 ± 0.06 |
| Cancer Type, n (%) | |||
| Breast | 6 (100%) | 6 (75%) | 4 (67%) |
| Colorectal | 0 (0%) | 2 (25%) | 2 (33%) |
| Cancer Diagnosis and Stage, n (%) | |||
| I | 4 (67%) | 1 (13%) | 3 (50%) |
| II | 2 (33%) | 2 (25%) | 0 (0%) |
| III | 0 (0%) | 4 (50%) | 3 (50%) |
| IV | 0 (0%) | 1 (13%) | 0 (0%) |
| Chemotherapy Type, n (%) | |||
| Taxane | 0 (0%) | 6 (75%) | 4 (67%) |
| Oxaliplatin | 0 (0%) | 2 (25%) | 2 (33%) |
| Diagnosis and Treatment Timing (weeks), mean ± SD | |||
| Time since diagnosis | 111.6 ± 97.1 | 27.7 ± 6.1a | 29.9 ± 6.7b |
| Time since completing chemotherapy | N/A | 3.7 ± 1.8 | 3.9 ± 1.3 |
| EORTC QLQ-CIPN20, m ± SD | |||
| Sensory | 98.1 ± 2.0 | 93.1 ± 5.4 | 54.3 ± 12.7b,c |
| Motor | 95.8 ± 6.5 | 89.1 ± 8.6 | 69.4 ± 16.2b,c |
| Autonomic | 100 ± 0 | 94.4 ± 7.9 | 75.9 ± 19.1 |
| Cognitive Function, m ± SD | |||
| GML_ter | 57.6 ± 21.4 | 55.4 ± 14.8 | 49.7 ± 18.0 |
| Average Walking Speed, m ± SD | |||
| Walking speed | 1.10 ± 0.17 | 0.89 ± 0.40 | 0.77 ± 0.32 |
EORTC QLQ-CIPN20 subscales are on a 0 to 100 scale with lower scores representing worse symptoms
Increases in GML_ter represent greater errors and worse executive function
P < 0.05 for difference between CON and -CIPN
P < 0.05 for difference between CON and +CIPN
P < 0.05 for difference between -CIPN and +CIPN
During the two 5-minute walking trials, bilateral lower extremity kinematics were measured using an MTP Series 2 motion capture system (Metria Innovation, Inc.; Milwaukee, WI). Reflective Moiré pattern markers were attached to bands that were wrapped securely around the pelvis, thighs, shanks, and adhered to the heels of the shoes. Relationships between these tracking markers and anatomical landmarks were defined using a custom digitization pointer. Kinematic trajectories were obtained using Visual3D (C-Motion, Inc.; Germantown, MD) and analyzed in MATLAB using custom scripts (Version 2015b; MathWorks, Inc.; Natick, MA). Marker data were recorded at 60 Hz and 4th order Butterworth lowpass filtered at 10 Hz.
The resulting kinematic data were further processed to yield a 12-dimensional state space. The first 30 s of each trial were omitted to exclude transient effects. Initial contact was identified as the maximum anterior position of the foot for each step. Data were trimmed to 145 strides for each trial for all participants and conditions, with each time series interpolated to 14,500 data points (nominally 100 samples per stride). The 12-dimensional state space was then defined using the 3D linear accelerations and 3D angular velocities of the pelvis center of mass that were time delay copied once by a constant time delay of 25 samples [16]. The state variables were demeaned and normalized to unit variance prior to analyzing to account for differences in units of variables [17].
The Floquet multiplier calculation followed previously described methods [12,18] (Electronic Supplemental Material). Briefly, 145 strides were used for all participants and conditions, where individual strides were defined using initial contact events and then normalized to 101 points (i.e., 0–100% gait cycle) that represented 101 Poincaré sections. The mean of the resulting 101 maxFM values was used as the estimate of orbital stability (i.e., maxFM_all). Additionally, the maxFM at initial contact was also included as an outcome variable (i.e., maxFM_IC) [18], as previous studies implementing nonlinear gait analyses have shown the most profound phase-dependent effects early in the stride cycle [19,20]. Increases in maxFM are associated with a decreasing rate of attenuating perturbations away from a fixed point, with maxFM > 1 associated with an orbitally unstable system (i.e., perturbations grow with subsequent cycles) [18].
A Groton Maze Learning Test was administered as part of a computerized test battery (Cogstate, Ltd., Melbourne, Australia) to gain insight into gross executive function deficits [21]. Particular interest was placed in executive function due to previous reports of deficits in this cognitive domain following chemotherapy [8] and its importance to gait [10]. The primary outcome for the current study was the total errors over five trials in attempting to learn the same hidden pathway that connected two targets (i.e., GML_ter). Increases in GML_ter correspond to worse performance.
Finally, participants also completed the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire – Chemotherapy-Induced Peripheral Neuropathy (EORTC QLQ-CIPN20), which assesses sensory, motor, and autonomic system interference [22]. Subscale scores (sensory, motor, autonomic) were transformed to a 0 to 100 scale, with lower scores indicating higher symptom severity. The EORTC QLQ-CIPN20 sensory subscale was used to separate participants who received chemotherapy into the -CIPN and +CIPN groups. This approach was chosen due to its alignment with the clinical practice of evaluating CIPN symptoms via similar sensory symptom questions. A cutoff of 80 for the sensory subscale score at the time the gait data were collected was chosen because scores greater than 80 have previously characterized 97% of a healthy, reference population [23].
Statistical Analysis
Kruskal-Wallis tests (nonparametric equivalent to an ANOVA) were used for both gait parameters to identify where at least one between-group difference was likely (p<0.05). Nonparametric tests were used as a conservative approach because they do not require data to be normally distributed, which is difficult to confirm in small sample sizes. Pairwise post-hoc comparisons between groups were then made with Wilcoxon Rank Sum tests on gait parameters with significant differences indicated by the Kruskal-Wallis test. Analyses were run separately on ST, DT, and change scores between conditions (i.e., DT-ST) for each gait parameter. The within-subject effect of the DT condition was assessed through Wilcoxon Signed Rank Sum tests. All analyses were performed in SAS (Version 9.4; SAS Institute, Inc.; Cary, NC). Effect sizes (ES) were estimated using Cohen’s d values, with 0.3–0.5, 0.5–0.8, and >0.8 interpreted as small, medium, and large effects, respectively [24].
Additionally, we investigated associations between executive function and orbital stability for the ST, DT, and dual-task cost (percent change between ST and DT) conditions. Spearman correlations were calculated in conjunction with a resampling with replacement bootstrapping procedure (1000 iterations) to determine the extent to which the point estimate for the small sample size available for this study was driven by a select few participants (MATLAB). Significance for all analyses was defined at α=0.05. We did not correct for multiple comparisons due to the exploratory nature of this study.
Results
Twenty individuals participated in the study after providing IRB-approved informed consent (Table 1). Participants were predominantly diagnosed with breast cancer (n=16 of 20), and those treated with chemotherapy were primarily treated with taxane-based chemotherapy (n=10 of 14). There were no significant differences between groups in age, mass, or height. The time since cancer diagnosis was greater in the CON group compared to both the -CIPN and +CIPN groups, which did not differ from each other. Consistent with the grouping criteria, the +CIPN group had significantly worse self-reported sensory symptoms of CIPN (Table 1).
Participants completed all tasks successfully with the following exceptions. Data for the DT gait condition for one CON participant was excluded due to the loss of foot markers midway through the trial. Therefore, for the CON group, data for six participants are included for ST gait, but only five participants for the DT and change score outcomes. Additionally, two +CIPN participants required a second attempt for the DT condition to successfully complete the task. The first attempt for these two participants was stopped early for safety as these participants quickly drifted toward the back of the treadmill upon starting the serial 7’s counting task. The second attempt for these participants was completed successfully, which may reflect a modification in their strategy to focus less on the serial 7’s task and/or focus more on gait compared to the first exposure to the condition.
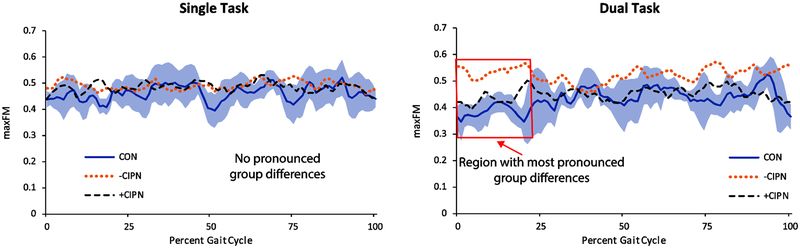
Significant differences in orbital stability between groups were evident during the DT gait condition (Table 2). The -CIPN group demonstrated decreased orbital stability during the DT condition at initial contact compared to the CON group (ES = 1.96, P = 0.019), with the +CIPN group showing a similar but smaller effect that did not reach statistical significance (ES = 1.35, P = 0.082). The -CIPN group also demonstrated decreased orbital stability compared to the +CIPN group during DT gait (ES = 1.43, P = 0.029). The decreased orbital stability for the -CIPN group appeared to be greatest early in stance, but a noticeable difference in maxFM can be seen for the DT condition across a majority of the gait cycle (Figure 2). These effects were not discernable for ST gait.
Table 2.
Group comparison of orbital gait stability parameters for single-task and dual-task conditions as well as the difference between conditions. Median (IQR)
| Gait Parameter |
Task | CON | −CIPN | +CIPN |
|---|---|---|---|---|
| ST | 0.48 (0.07) | 0.46 (0.12) | 0.51 (0.17) | |
| maxFM_all | DT | 0.42 (0.03) | 0.51 (0.16) | 0.45 (0.07) |
| DT-ST | −0.04 (0.04) | 0.04 (0.07) | −0.04 (0.18) | |
| ST | 0.45 (0.06) | 0.48 (0.06) | 0.45 (0.22) | |
| maxFM_IC | DT | 0.35 (0.04) | 0.56 (0.20)a | 0.42 (0.05)b |
| DT-ST | −0.08 (0.03) | 0.09 (0.14)a | −0.06 (0.19) |
P < 0.05: -CIPN vs. CON
P < 0.05: +CIPN vs. -CIPN
IQR: interquartile range
maxFM_all: maximum Floquet multiplier averaged across the entire gait cycle
maxFM_IC: maximum Floquet multiplier at the Poincaré section of initial contact
All values are in arbitrary units
Figure 2.
Maximum Floquet multiplier (maxFM) across the gait cycle during single-task (left) and dual-task (right) gait. Lines represent group means and shaded region corresponds to ± 1SD for the CON group. The most pronounced differences between groups are that the -CIPN group had higher maxFM estimates early in the gait cycle for the dual-task condition, indicating lower orbital stability.
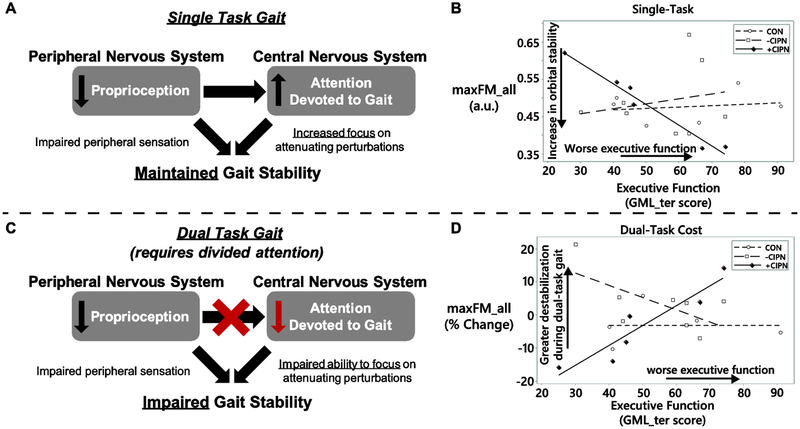
Associations between executive function and orbital stability were only seen in the +CIPN group (Table 3, Figure 3). During ST gait, decreased executive function was associated with improved orbital stability for both maxFM_all (ρ = −0.94; P = 0.017) and maxFM_IC (ρ = −1.00; P = 0.003) in the +CIPN group. Additionally, worse executive function was associated with an exacerbated dual task cost on orbital stability for participants in the +CIPN group (maxFM_all: ρ = 0.94; P = 0.017).
Table 3.
Spearman correlations between executive function and orbital gait stability. Correlations presented for single-task, dual task, and dual-task cost. Spearman’s ρ (95% confidence interval)
| Gait Parameter |
Group | Executive Function (GML_ter) | ||
|---|---|---|---|---|
| Single Task | Dual Task | Dual-Task Cost | ||
| CON | −0.03 (−0.39, 0.41) | −0.10 (−0.54, 0.43) | 0.10 (−0.49, 0.53) | |
| maxFM_all | −CIPN | −0.06 (−0.34, 0.19) | −0.20 (−0.46, 0.06) | −0.60 (−0.78, −0.32) |
| +CIPN | −0.94 (−1.05, −0.77)* | 0.43 (0.00, 0.80) | 0.94 (0.78, 1.05)* | |
| CON | −0.49 (−0.84, −0.04) | −0.20 (−0.72, 0.29) | 0.50 (−0.09, 0.8) | |
| maxFM_IC | −CIPN | 0.16 (−0.12, 0.38) | 0.08 (−0.20, 0.35) | 0.17 (−0.15, 0.52) |
| +CIPN | −1.00 (−1.00, −1.00)** | −0.54 (−0.87, −0.16) | 0.83 (0.53, 1.03) | |
95% confidence intervals obtained from bootstrapping procedure
P < 0.05
P < 0.01
Figure 3.
Proposed theoretical framework to interpret the increased dual-task cost for orbital stability in participants with CIPN sensory symptoms and poorer executive function. A. Peripheral nervous system impairments are commonly associated with CIPN. These impairments may result in a compensatory increase in cognitive attention devoted to gait to accommodate degraded afferent sensory information. Greater concentration on gait may correspond to maintained or increased attenuation of perturbations during gait, which would represent a potential increase in orbital stability. B. Experimental data showing increased orbital stability in +CIPN participants (solid markers and line; ρ = −0.94 (95% CI: −1.05, −0.77)) with worse executive function during single-task gait. C. When a simultaneous cognitive task is introduced (i.e., a dual task), the ability to compensate for degraded afferent information via increased attention is hindered. Individuals with cognitive impairments may be preferentially impacted. A consequence of the degraded afferent information along with divided attention may be a decrease in orbital stability for individuals with combined peripheral and central nervous system impairments. D. Experimental data showing increased dual-task cost (% change from single- to dual-task gait) in orbital stability for +CIPN participants with worse executive function (ρ = 0.94 (95% CI: 0.78, 1.05)). The aforementioned relationships were not detected in the CON or -CIPN groups (hollow markers and dashed lines), which is consistent with an attentionally-demanding compensatory strategy in +CIPN group being associated with the presence of sensory symptoms of CIPN. As everyday activities are performed in the presence of a number of potential concurrent demands and distractions, dual-task assessments may provide insight into physiologically-relevant demands and behavior that exist outside of the laboratory.
Discussion
Falls are a major concern for the long-term quality of life of a number of populations including cancer survivors [1,2,5], and many falls occur during ambulation. This exploratory study aimed to decouple gait impairments associated with central and peripheral nervous system impairments using commonly reported adverse effects of chemotherapy treatment as a model. The results of this study partially support the study hypotheses that (1) impaired peripheral sensation related to CIPN would be associated with decreased orbital stability during gait, and (2) participants with CIPN and cognitive impairments would demonstrate worsened gait impairments during dual-task walking. Participants with combined peripheral sensory and cognitive impairments demonstrated worsened gait impairments during DT walking. Isolated impairments in peripheral sensation or cognitive function did not significantly associate with gait stability. This initial work supports the potential importance of monitoring sensory and cognitive deficits when assessing for potential contributing factors to impaired gait in cancer survivors and potentially other populations. Future studies that quantify gait stability and also prospectively track falls in this population are needed to determine the fall-risk relevance of the factors associated with gait instability in this study.
In our sensory symptomatic group (+CIPN), we observed that relatively worse executive function was associated with improved orbital stability during ST gait, but also a greater decrease in orbital stability during DT gait. Our working theory is that peripheral nervous system impairments are compensated by increased central nervous system involvement, when possible. Under this theory, participants with self-reported sensory symptoms of neuropathy increase the amount of attention devoted to attenuate perturbations during ST gait to compensate for impaired peripheral sensation and maintain orbital stability (Figure 3A, 3B). During DT gait, this strategy would likely be impaired in individuals with diminished executive function because they must divide attention between the cognitive task and the locomotor task (Figure 3C, 3D). The resulting effect would be a diminished ability to compensate for impaired sensory afferents with increased attention. This proposed effect is consistent with the increased dual-task cost associated with worse executive function in our +CIPN group.
The proposed theory is consistent with several preceding studies. Dingwell et al. previously reported subject-dependent decreases in orbital stability during DT treadmill walking in healthy participants, suggesting that additional cognitive load can be associated with impaired orbital stability in certain individuals [25]. Furthermore, Dingwell et al. reported a small increase in orbital stability at the knee of participants with diabetic peripheral neuropathy (DPN) compared to healthy controls [26], providing an instance of peripheral sensory deficits being associated with improved orbital stability during single-task gait. Finally, Sloot et al. reported an increase in orbital stability during a vestibular-impaired single-task gait condition [27]. While the authors interpreted their findings as a limitation of the Floquet multiplier analysis, our proposed theory of a central nervous system compensation for peripheral nervous system impairment may provide an alternative explanation. Notably, executive function ability has previously been used to explain altered dual-task gait in concussed and neurologically-impaired populations when using other gait stability measures [11,28,29]. Further studies that verify this behavior in a larger sample size and assess attentional focus during single- and dual-task gait are needed to evaluate this theory.
Additionally, prior chemotherapy treatment was negatively associated with gait stability in our -CIPN group. These findings provide initial insight into the multifactorial challenges that burden cancer survivors who receive neurotoxic chemotherapy. The -CIPN group demonstrated decreased orbital stability during DT gait (ES = 1.96), with a smaller effect size (ES = 1.35) observed for the +CIPN group. Given two +CIPN participants failed their first attempt and required second attempts for the DT gait condition, our DT gait stability estimates for the +CIPN group may be artificially improved relative to their naïve DT exposure that was unsuccessful. These findings suggest that chemotherapy treatment may be associated with impaired gait stability, however the current study did not provide evidence for an isolated association between CIPN and orbital stability.
While this study provides novel contributions to better understand the roles of central and peripheral nervous function in gait stability, there are several limitations that should be considered. There was no correction for multiple comparisons. Therefore, caution should be taken in these findings, particularly those with marginal P-values, as the risk of Type I error is elevated. We conservatively used nonparametric methods for hypothesis testing and estimating correlations due to our small sample size. Larger sample sizes are necessary to gain further confidence in our findings and identify whether several trends found here represent significant differences. Additionally, larger sample sizes would enable subgroup evaluation into potential differences in behavior between various cancer types, chemotherapy drug classes, patient age, etc. as well as statistically control for potential confounding factors such as time since diagnosis. Future studies of the same nature may be strengthened by also implementing a DT condition during overground walking and recording the accuracy of the DT performance during gait and sitting. Implementing an overground DT approach would enable common real-world gait adaptations that are suppressed during treadmill walking, such as slowed gait speed [30]. While our fixed-speed constraint on the treadmill deviates from adaptations that may naturally occur outside of the laboratory, our experimental protocol provides insight into the robustness of individuals’ control schemes when subjected to a challenging dual-task condition.
Conclusion
This exploratory study provides insight into the compounding destabilizing effect that can occur during cognitively-challenging gait in individuals with concomitant impairments in peripheral sensation and cognitive function using a group of cancer survivors. These findings warrant further evaluation in a larger cohort to understand their clinical relevance and to continue to better delineate the contributions that peripheral and central nervous system factors have on orbital gait stability.
Supplementary Material
Highlights.
Executive plus sensory dysfunction associated with maintained single-task stability
Executive plus sensory dysfunction associated with impaired dual-task stability
A cognitive compensatory strategy during single-task gait may explain these results
Asymptomatic survivors had decreased orbital stability following chemotherapy
Acknowledgements
This research was supported by the National Cancer Institute (Grant No. R03 CA182165) and the National Science Foundation Graduate Research Fellowship Program (Grant No. DGE-1343012). Additional financial support for this work was provided by Ohio State University’s Center for Clinical and Translational Science (partial support for Xueliang Pan) and Graduate School (partial research support for Scott Monfort). The authors would like to thank Nelson Glover for his contributions in preparing the gait data for analysis, and Lynette Mesi for her contributions to patient recruitment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None.
Location Research was Conducted: OSU Sports Biomechanics Laboratory, The Ohio State University, Martha Morehouse Medical Plaza, 2050 Kenny Road, Columbus, Ohio, 43221, USA.
References
- [1].Gewandter JS, Fan L, Magnuson A, Mustian KM, Peppone LJ, Heckler C, Hopkins J, Tejani M, Morrow GR, Mohile SG, Falls and functional impairments in cancer survivors with chemotherapy-induced peripheral neuropathy (CIPN): A University of Rochester CCOP study, Support. Care Cancer 21 (2013) 2059–2066. doi: 10.1007/s00520-013-1766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tofthagen C, Overcash J, Kip K, Falls in persons with chemotherapy-induced peripheral neuropathy., Support. Care Cancer 20 (2012) 583–589. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ocean AJ, Vahdat LT, Chemotherapy-induced peripheral neuropathy: Pathogenesis and emerging therapies, Support. Care Cancer 12 (2004) 619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- [4].Monfort SM, Pan X, Patrick R, Ramaswamy B, Wesolowski R, Naughton MJ, Loprinzi CL, Chaudhari AM, Lustberg MB, Gait, balance, and patient-reported outcomes during taxane-based chemotherapy in early-stage breast cancer patients., Breast Cancer Res. Treat (2017). doi: 10.1007/s10549-017-4230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Winters-Stone KM, Hilton C, Luoh S-W, Jacobs P, Faithfull S, Horak FB, Comparison of physical function and falls among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. Subcategory: Long-term Complications/Sequelae of Treatment (Noncancer), in: J Clin Oncol, 2016: p. 130.26598755 [Google Scholar]
- [6].Marshall TF, Zipp GP, Battaglia F, Moss R, Bryan S, Chemotherapy-induced- peripheral neuropathy, gait and fall risk in older adults following cancer treatment, J. Cancer Res. Pract (2017) 20. doi: 10.1016/j.jcrpr.2017.03.005. [DOI] [Google Scholar]
- [7].Monfort SM, Pan X, Loprinzi CL, Lustberg MB, Chaudhari AM, Impaired Postural Control and Altered Sensory Organization During Quiet Stance Following Neurotoxic Chemotherapy : A Preliminary Study, Integr. Cancer Ther 18 (2019) 1–8. doi: 10.1177/1534735419828823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahles TA, Saykin AJ, Candidate mechanisms for chemotherapy-induced cognitive changes., Nat. Rev. Cancer 7 (2007) 192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jim HSL, Phillips KM, Chait S, Faul LA, Popa MA, Lee YH, Hussin MG, Jacobsen PB, Small BJ, Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy, J. Clin. Oncol 30 (2012) 3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Amboni M, Barone P, Hausdorff JM, Cognitive contributions to gait and falls: Evidence and implications, Mov. Disord 28 (2013) 1520–1533. doi: 10.1002/mds.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yogev-Seligmann G, Hausdorff JM, Giladi N, The role of executive function and attention in gait, Mov. Disord 23 (2008) 329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bruijn SM, Meijer OG, Beek PJ, van Dieen JH, Assessing the stability of human locomotion: a review of current measures., J. R. Soc. Interface 10 (2013) 20120999. doi: 10.1098/rsif.2012.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Granata KP, Lockhart TE, Dynamic stability differences in fall-prone and healthy adults, J. Electromyogr. Kinesiol 18 (2008) 172–178. doi: 10.1016/j.jelekin.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lockhart TE, Liu J, Differentiating fall-prone and healthy adults using local dynamic stability., Ergonomics. 51 (2008) 1860–72. doi: 10.1080/00140130802567079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jordan K, Challis JH, Newell KM, Walking speed influences on gait cycle variability, Gait Posture. 26 (2007) 128–134. doi: 10.1016/j.gaitpost.2006.08.010. [DOI] [PubMed] [Google Scholar]
- [16].Bruijn SM, Ten Kate WRT, Faber GS, Meijer OG, Beek PJ, Van Dieën JH, Estimating dynamic gait stability using data from non-aligned inertial sensors, Ann. Biomed. Eng 38 (2010) 2588–2593. doi: 10.1007/s10439-010-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kang HG, Dingwell JB, A direct comparison of local dynamic stability during unperturbed standing and walking, Exp. Brain Res. 172 (2006) 35–48. doi: 10.1007/s00221-005-0224-6. [DOI] [PubMed] [Google Scholar]
- [18].Arellano CJ, Layne CS, O’Connor DP, Scott-Pandorf M, Kurz MJ, Does load carrying influence sagittal plane locomotive stability?, Med. Sci. Sports Exerc 41 (2009) 620–627. doi: 10.1249/MSS.0b013e31818a0ea4. [DOI] [PubMed] [Google Scholar]
- [19].Ihlen EAF, Goihl T, Wik PB, Sletvold O, Helbostad J, Vereijken B, Phase-dependent changes in local dynamic stability of human gait, J. Biomech 45 (2012) 2208–2214. doi: 10.1016/j.jbiomech.2012.06.022. [DOI] [PubMed] [Google Scholar]
- [20].Fino PC, Mancini M, Curtze C, Nutt JG, Horak FB, Gait stability has phase-dependent dual-task costs in Parkinson’s disease, Front. Neurol 9 (2018). doi: 10.3389/fneur.2018.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pietrzak RH, Maruff P, Mayes LC, Roman SA, Sosa JA, Snyder PJ, An examination of the construct validity and factor structure of the Groton Maze Learning Test, a new measure of spatial working memory, learning efficiency, and error monitoring, Arch. Clin. Neuropsychol 23 (2008) 433–445. doi: 10.1016/j.acn.2008.03.002. [DOI] [PubMed] [Google Scholar]
- [22].Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lantéri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R, The development of an EORTC quality of life questionnaire to assess chemotherapy- induced peripheral neuropathy: The QLQ-CIPN20, Eur. J. Cancer 41 (2005) 1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- [23].Mols F, van de Poll-Franse LV, Vreugdenhil G, Beijers AJ, Kieffer JM, Aaronson NK, Husson O, Reference data of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-CIPN20 Questionnaire in the general Dutch population, Eur. J. Cancer 69 (2016) 28–38. doi: 10.1016/j.ejca.2016.09.020. [DOI] [PubMed] [Google Scholar]
- [24].Cohen J, A Power Primer, Quant. Methods Psychol 112 (1992) 155–159. [DOI] [PubMed] [Google Scholar]
- [25].Dingwell JB, Robb RT, Troy KL, Grabiner MD, Effects of an attention demanding task on dynamic stability during treadmill walking., J. Neuroeng. Rehabil 5 (2008) 12. doi: 10.1186/1743-0003-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dingwell JB, Kang HG, Marin LC, The effects of sensory loss and walking speed on the orbital dynamic stability of human walking, J. Biomech 40 (2007) 1723–1730. doi: 10.1016/j.jbiomech.2006.08.006. [DOI] [PubMed] [Google Scholar]
- [27].Sloot LH, Van Schooten KS, Bruijn SM, Kingma H, Pijnappels M, van Dieen JH, Sensitivity of local dynamic stability of over-ground walking to balance impairment due to galvanic vestibular stimulation, Ann. Biomed. Eng 39 (2011) 1563–1569. doi: 10.1007/s10439-010-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Parker TM, Osternig LR, Lee HJ, Van Donkelaar P, Chou LS, The effect of divided attention on gait stability following concussion, Clin. Biomech 20 (2005) 389–395. doi: 10.1016/j.clinbiomech.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [29].Lamoth CJ, van Deudekom FJ, van Campen JP, Appels B. a, de Vries OJ, Pijnappels M, Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people, J. Neuroeng. Rehabil 8 (2011) 2. doi: 10.1186/1743-0003-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Muir SW, Gopaul K, Montero Odasso MM, The role of cognitive impairment in fall risk among older adults: A systematic review and meta-analysis, Age Ageing. 41 (2012) 299–308. doi: 10.1093/ageing/afs012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.