Figure 4 |. Structure-based engineering of a superior analogue of FGF21 and the mechanism of endocrine FGF signaling.
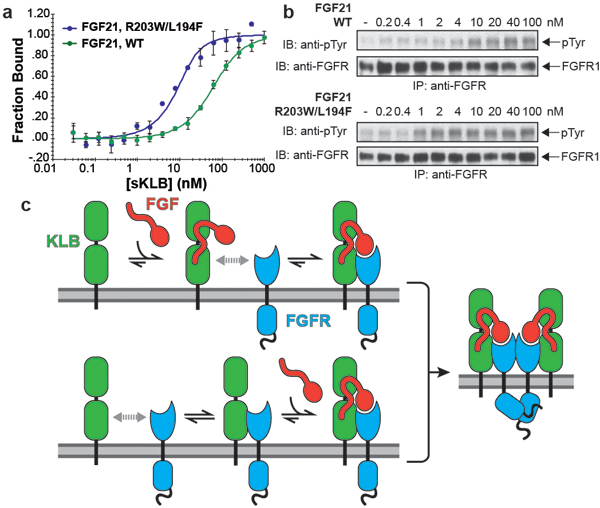
a, b, Enhanced binding affinity (a) and bioactivity (b) of an FGF21 mutant. MST binding measurements of FGF21 carrying a double L194F/R203W mutations in FGF21CT reveal approximately 10-fold increase in binding affinity to sKLB with a KD of 3.4 ± 1.3 nM and approximately 10-fold enhanced potency for stimulation of FGFR1c tyrosine phosphorylation. The dots and error bars for each graph in panel a denote means and variations of ΔFnorm (n = 3 independent samples). Individual experimental data are plotted in Supplementary Fig. 2. c, A ‘Zip code’-like mechanism for β-Klotho dependent FGF21 stimulation of FGFR1c. In the cell membrane of unstimulated cells β-Klotho and FGFR1c monomers are in equilibrium with FGFR/β-Klotho heterodimers. Due to reduced dimensionality, the binding of FGF21 to β-Klotho via FGF21 C-tail and bi-valent binding of the FGF core of FGF21 to two FGFR1c molecules will shift the equilibrium towards formation of a FGF21/FGFR1c/β-Klotho ternary complexes, resulting in stimulation of tyrosine kinase activity and cell signaling via FGFR1c. In addition, β-Klotho functions as a primary high affinity receptor for FGF21 and FGFR1c functions as a catalytic subunit that mediate receptor dimerization and intracellular signaling.
