Extended Data Figure 3 |. Unique structural features of sKLB.
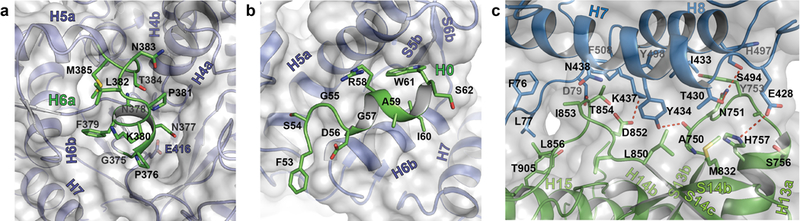
a, Interaction of H6a (green) with the pseudo-substrate binding pocket in D1 of sKLB. Glu416, the ‘catalytic’ glutamic acid residue in D1, located on the bottom of the pocket is also highlighted. b, Interaction of H0 (green) with the nearby structural elements in D1 of sKLB. c, Interface between D1 (skyblue) and D2 (green) of sKLB highlighting amino acids and structural elements as well as polar interactions (red dotted lines) between the domains.
