Fig. 5.
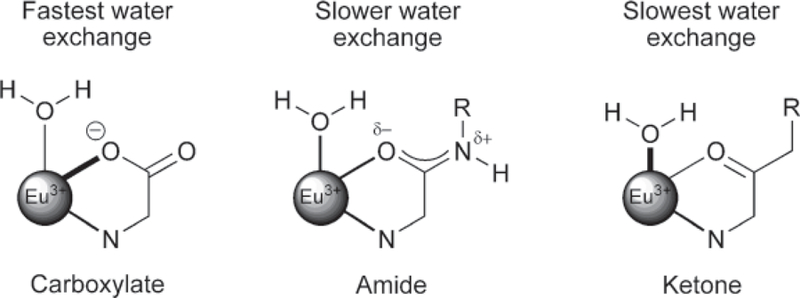
Electron delocalization from ligand oxygen donors to the lanthanide ion decreases in the donor series, carboxylates > amides > ketones. This results in the weakest Eu3+-water interaction in the carboxylate systems and faster water exchange. In the extreme, ketone-type ligands would contribute the least electron density, and thereby promote the strongest Eu3+-water interaction and slowest water exchange. Amide-containing systems fall between these extremes.
