Abstract
The efficacy of asparaginase in acute lymphoblastic leukemia (ALL) is dependent on depletion of asparagine, an essential amino acid for ALL cells. The target level of plasma asparaginase activity to achieve asparagine depletion has been between 0.05–0.4 IU/mL. COG AALL07P4 examined the asparaginase activity and plasma and CSF asparagine concentration of pegaspargase when given intravenously in the treatment of NCI high risk ALL. Matched plasma asparaginase/asparagine levels of the clearance of 54 doses of pegaspargase given in induction or consolidation demonstrated that all patients who had a plasma asparaginase level >0.02 IU/mL had undetectable plasma asparagine. No difference was observed in CSF asparagine levels associated with matched plasma asparaginase levels of 0.02–0.05 vs. 0.05–0.22 IU/mL (p=0.25). Our data suggest that a plasma asparaginase activity level of 0.02 IU/mL can effectively deplete plasma asparagine. The data also indicate that the 95%CI for plasma asparagine depletion after a pegaspargase dose is 22–29 days.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy.[1] Asparaginase is a critical component of the multi-agent chemotherapy that has resulted in a significant improvement in the outcomes of children with ALL.[2] Asparagine is an essential amino acid for lymphoblasts, which are deficient in asparagine synthetase. Depletion of asparagine interferes with protein synthesis and induces cell death.[3,4] Although asparaginase does not cross the blood brain barrier, studies have demonstrated cerebrospinal fluid (CSF) asparagine depletion after treatment as a results of plasma asparagine depletion.[5]
Previously published reports have estimated the target level of plasma asparaginase threshold required to induce and maintain asparagine depletion to be in the 0.05 to 0.4 IU/mL range, information of critical importance in maximizing the therapeutic impact of this agent.[5–14] The U.S. FDA has used trough plasma asparaginase levels ≥0.1 IU/mL to determine efficacy as part of the approval of Erwinia asparaginase.[15] A more specific, accurate threshold would allow optimal planning for when to measure trough levels, in conjunction with known pharmacokinetics (PKs) of the specific asparaginase product.
We report on a subset of patients on Children’s Oncology Group (COG) trial AALL07P4 who received a standardized dose (2500 IU/m2) of pegaspargase intravenously (IV) during induction and consolidation as part of an augmented BFM chemotherapy regimen for the treatment of NCI high risk (HR) ALL.[16] We performed more detailed studies of plasma asparaginase activity and plasma and CSF asparagine levels in order to determine the optimal activity threshold required for asparagine depletion.
Patients, Materials, and Methods
Patients
Eligible patients (ages 1–30.99 years) had newly diagnosed HR B-ALL (age ≥10 years and/or initial white blood cell count ≥50,000/µL).[17] Key exclusion criteria included patients with Down syndrome, testicular leukemia, prior cytotoxic chemotherapy, pregnancy, and breastfeeding females.
Institutional review board approval was obtained at each participating institution prior to patient enrollment. Written informed consent and assent (when appropriate) were obtained from each patient and/or their parent/guardian prior to initiation of therapy. This trial was registered at www.clinicaltrials.gov as #NCT00671034.
Study Design
All patients received a modified COG augmented BFM chemotherapy regimen with the asparaginase preparation (calasparagse pegol or pegaspargase) assigned at study enrollment by randomization, as previously reported.[16] Initial PK/PD has been previously reported [16] and in this report we present more detailed PK/PD analysis focused on the individual patient level data obtained on the subset of fifty-four patients who were randomized to receive 2500 IU/m2 of pegaspargase IV over 1 hour on Day 4 of induction and Day 15 of consolidation. This manuscript examines plasma asparaginase activity PK and plasma and CSF asparagine concentrations (pharmacodynamics; PD) following a total of 86 full doses given to 48 patients for whom adequate PK/PD samples for analysis were collected. In addition, three patients who received less than the intended dose, due to hypersensitivity reactions, and had matched asparaginase and plasma asparagine levels data are also included.
Pharmacokinetics/Pharmacodynamics
Plasma asparaginase activity was determined by a validated enzymatic-coupled assay with a lower limit of detection of 0.013 IU/mL.[16] Asparagine was determined using validated reverse-phase high-performance-liquid chromatography and double mass spectrometry that has a lower limit of quantification of 0.05 µg/mL.[16] Both were performed at a central laboratory [Frontage Laboratories (Malvern, PA)] as previously reported.[16] This trial was conducted with strict on-site instruction and monitoring of the processing of blood samples to minimize ex vivo hydrolysis of asparagine including: placing specimens on ice within 1 minute of collection, centrifugation at 4°C (within 5 minutes of blood collection), subsequent separation of plasma, addition of SERAPREP® to the aliquot to be used for asparagine analysis, and flash freezing with dry ice and storage at −70°C until analysis. The maximum total processing time was required to be less than 15 minutes.[16]
CSF Asparagine / Plasma Asparaginase Comparisons
Protocol-specified administration of intrathecal chemotherapy allowed CSF asparagine levels to be obtained within a few hours of plasma asparaginase levels. These CSF asparagine/plasma asparaginase pairs were used to evaluate the relative contribution of ex vivo deamination (hydrolysis, degradation) of asparagine in plasma samples. These pairs were also used to evaluate the validity of the plasma asparaginase levels below 0.05 IU/mL in comparison with higher levels.
Results
Patient characteristics
Between October 2008 and December 2010, 54 eligible patients ages 1.08–23.49 years (mean age 10.74 years; 57% male) were randomized to receive 2500 IU/m2 of pegaspargase (Oncaspar© , Shire Pharmaceuticals, Lexington, MA) IV as part of their NCI HR ALL therapy. Of these 54 patients, 48 had adequate PK disappearance curves and 38 had paired plasma asparaginase-asparagine PK/PD specimens and were considered PK evaluable. Demographic characteristics have been previously reported.[16]
Plasma asparaginase PK
Figure 1 and Supplementary Fig. S1 depict the plasma asparaginase activity levels in 48 patients evaluable for PK. The clearance of pegaspargase was multiphasic with a rapid decline in plasma asparaginase activity during the first day, a slower decline during days 1–7, followed by a further slow decline during the second week, and an increasingly more rapid decline thereafter (Fig. 1A). The acceleration of clearance was most notable after day 21 when the activity fell below 0.02 IU/mL (Fig. 1A, Supplementary Fig. S1).
Figure 1. (A) 619 Asparaginase Levels after 86 Induction and Consolidation Doses of Pegaspargase, 2500 IU/m2, in 48 Patients, and (B) 95% Confidence Intervals of 480 Values on Days 1–28.
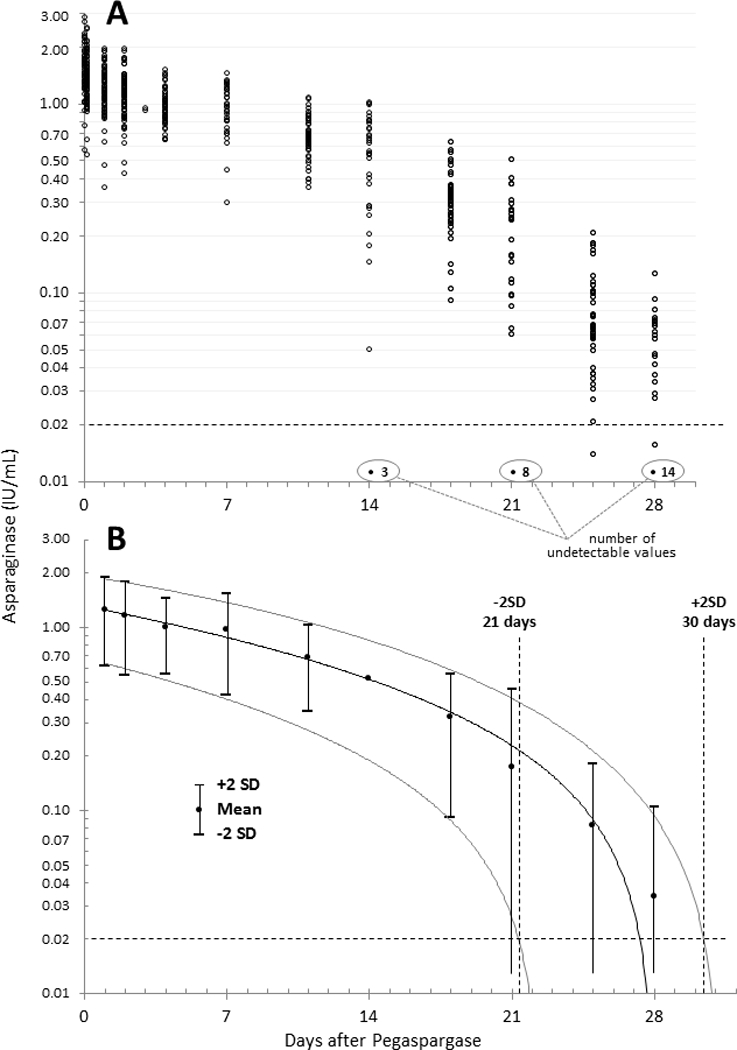
Regressions are 2° polynomials were determined with 25 undetectable values (all during days 14–28) assumed to be zero (0). The horizontal, dashed line indicates when, according to the results of this study, asparagine is repleted after asparaginase declines below this level. The polynomial regression is virtually identical if undetectable levels were defined as 0.001 or 0.010 IU/mL
Plasma asparaginase activity and corresponding plasma asparagine levels
Table 1 lists terminal paired plasma asparaginase and plasma asparagine levels for all asparaginase levels below 0.10 IU/mL following 51 doses of pegaspargase in 38 HR ALL patients administered during induction or consolidation. The mean (95% C.I.) plasma asparagine level before any asparaginase therapy was 7.82 (1.92–13.72) µg/mL (determined in all 152 patients treated on the clinical trial, including those randomized to calasparagse pegol). All patients who had plasma asparaginase activity greater than 0.02 IU/mL but less than 0.10 IU/mL had no plasma asparagine detected in samples obtained at the same time, regardless of the interval after dose administration. Two patients whose terminal plasma asparaginase level was detectable above the lower limit of asparaginase detection of 0.013 IU/mL but below 0.02 IU/mL had detectable plasma asparagine levels. Figure 2 demonstrates the PK/PD relationship in these 2 patients. After administration of 2500 IU/m2 pegaspargase IV, patient A rapidly developed a peak plasma asparaginase activity of greater than 1 IU/mL which subsequently declined over the depicted 25 day period. On all days up to and including day 18, plasma asparagine was undetectable. On day 25, plasma asparagine returned to this patient’s baseline level of 6.74 µg/mL when the plasma asparaginase activity was 0.014 IU/mL.
Table 1.
Paired terminal clearance of plasma asparaginase activity and asparagine levels in 38 children and adolescents with HR ALL who received 51 doses of pegaspargase, 2500 IU/m2, in induction or consolidation.
| Plasma Asparaginase Activity(IU/ml) | Number of paired samples with | |
|---|---|---|
| Undetectable Asparagine | Detectable Asparagine | |
| 0.013 to <0.02 | 0 | 2 |
| 0.02 to <0.05 | 16 | 0 |
| 0.05 to <0.10 | 36 | 0 |
Figure 2. The Two Patients with Asparagine Repletion at the Lowest Detectable Asparaginase Levels.
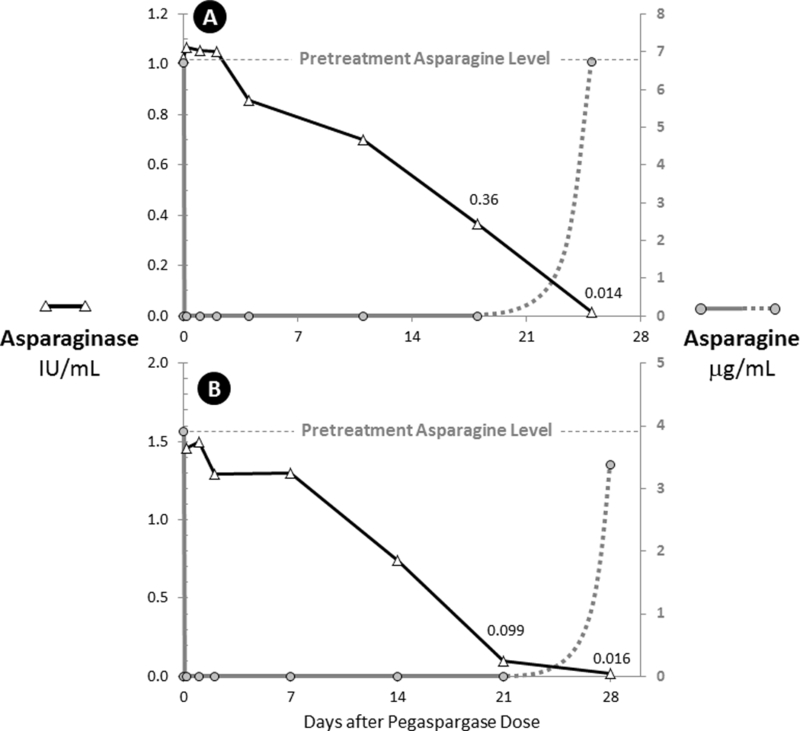
A. Induction dose in a patient whose plasma asparagine returned to pretreatment levels when the plasma asparaginase level was 0.014 IU/mL.
B. Consolidation dose in a patient whose plasma asparagine returned to pretreatment levels when the plasma asparaginase level was 0.016 IU/mL.
Similarly, patient B rapidly developed a peak plasma asparaginase activity of greater than 1.5 IU/mL which subsequently declined over the depicted 28 day period. On all days up to and including day 21, plasma asparagine was undetectable. On day 28, plasma asparagine returned to near this patient’s baseline level of 3.37 µg/mL when the plasma asparaginase activity was 0.016 IU/mL.
Figure 3 demonstrates the PK/PD relationship in three patients who had their pegaspargase administration terminated due to a hypersensitivity reaction during the infusion. Their peak plasma asparaginase levels never exceeded 0.36 IU/mL but they still achieved plasma asparagine depletion. Patient C, D, E received 17%, 3% and 31% of the prescribed dose, respectively. Despite the minimal dose of pegaspargase received, plasma asparagine levels were undetectable in all three patients with patient E remaining undetectable for 18 days when plasma asparaginase levels were between 0.19 to 0.26 IU/mL.
Figure 3. Three Patients with Complete Asparagine Depletion at Asparaginase Levels between 0.08 and 0.30 IU/mL.
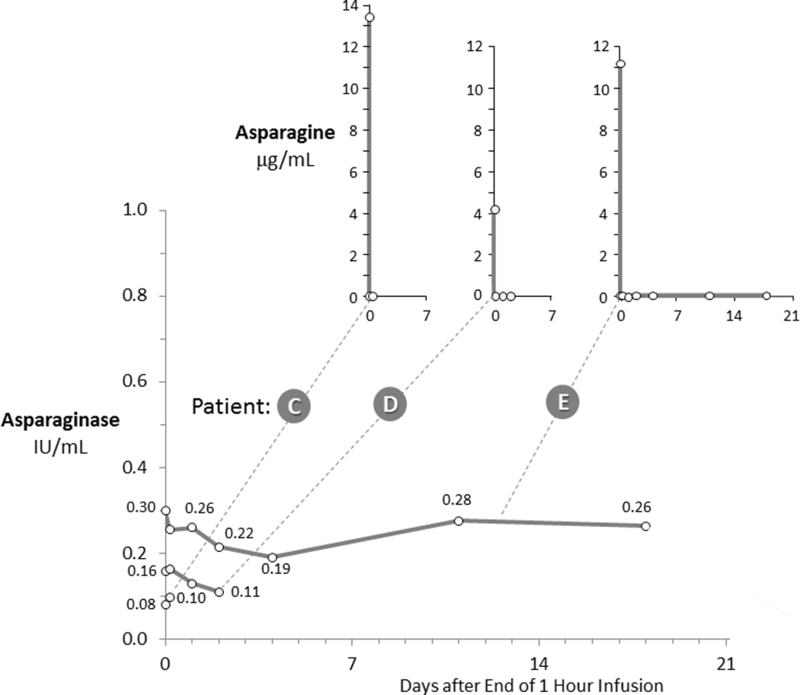
The insets depict paired asparagine levels for each patient’s asparaginase levels shown in the primary graph. Patients C, D and E received 17%, 3% and 31%, respectively, of the planned 2,500 IU/m2 dose due to hypersensitivity reactions.
Plasma asparaginase activity and corresponding CSF asparagine levels
In order to remove the potential effects of ex-vivo hydrolysis, we focused on all available CSF asparagine / plasma asparaginase pairs which were obtained between 15–43 days after asparaginase administration. The early time point of 15 days was chosen in order to allow sufficient time for the CSF asparagine decline to be fully effected (data not shown), while the upper limit of the time range (43 days) was the longest interval monitored after pegaspargase administration.
Figure 4 shows all 40 CSF asparagine/plasma asparaginase paired values that were obtained between 15 and 43 days after pegaspargase dosing. Of the 32 pairs with detectable asparaginase, 10 had plasma asparaginase levels between 0.02 and 0.05 IU/ml. There was no difference between CSF asparagine levels associated with plasma asparaginase levels of 0.02–0.05 and 0.05–0.22 IU/mL (p = 0.25), and their regressions are virtually identical (slopes of −0.25 and −0.20, p = 0.91 and p = 0.71) (Fig. 4). All 10 available paired CSF asparagine/plasma asparaginase samples with asparaginase levels <0.05 IU/mL 15 to 43 days after pegaspargase administration were within the 95% confidence interval of the 22 paired levels with plasma asparaginase between 0.05 IU/mL and 0.02 IU/mL (Fig. 4).
Figure 4. CSF Asparagine Concentration 15 to 43 Days after Pegaspargase, 2,500 IU/m2 IV, as a Function of Plasma Asparaginase activity, Based on All Available Paired Samples.
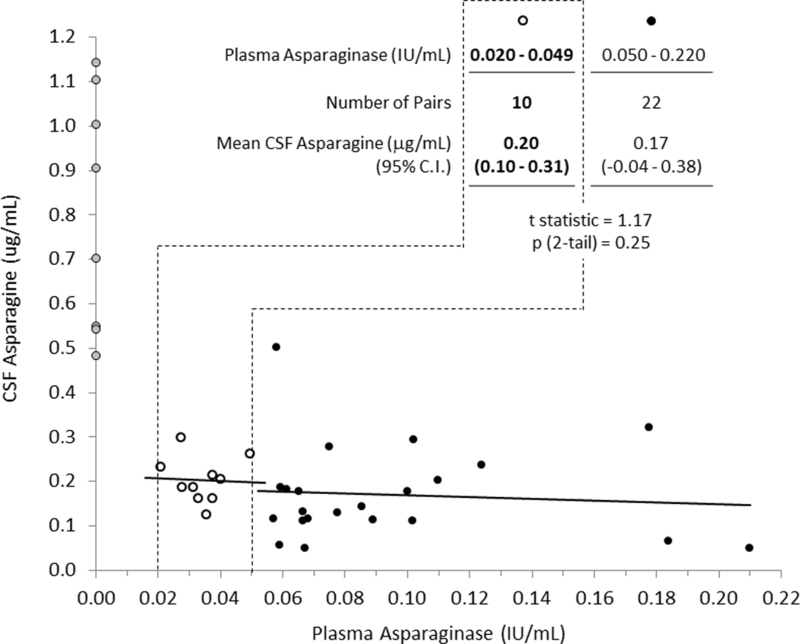
Gray circles are 8 pairs with undetectable plasma asparaginase (<0.013 IU/mL). Solid black dots outlined by a black dashed line are 10 pairs with plasma asparaginase activity between 0.02 and 0.05 IU/mL and their linear regression are denoted in red. Open circles represent 22 pairs with plasma asparaginase activity >0.05 IU/mL.
Duration of asparagine depletion
The terminal clearance of plasma asparaginase after 2500 IU/m2 of pegaspargase was evaluated using 480 plasma activity levels on days 1–28 inclusive in 48 patients. Assuming that 0.02 IU/mL asparaginase activity is the threshold for asparagine repletion, the 95% confidence intervals for each day evaluated indicates that 96% of patients treated with 2,500 IU/m2 pegaspargase during induction or consolidation have asparagine depletion for 22 to 29 days (Fig. 1B).
Comparison of asparaginase PK after 1st and 2nd doses
Of 32 patients who completed the pegaspargase infusions and had PK performed after both induction and consolidation doses (Doses #1 and #2, respectively), 21 (63%) had the same profile of asparaginase activity after dose #2, 5 (16%) had higher levels (gray highlighted), and 6 (18%) had evidence for delayed accelerated clearance after dose #2. Only one patient (3%) had evidence of silent inactivation (solid black outline). (Supplementary Figure 2)
Discussion
Matched plasma asparaginase/asparagine levels of the terminal clearance of pegaspargase in children with NCI HR ALL demonstrated that all patients who had plasma asparaginase activity greater than 0.02 IU/mL had undetectable plasma asparagine. In all but three cases there were high peak levels of asparaginase activity (>0.5 IU/mL in all cases and >1 IU/mL in over 90% of cases) and during the terminal decay phase there was no detectable plasma asparagine as long as the asparaginase activity level remained above 0.02 IU/mL. In three cases the infusions were interrupted due to allergic reactions (3–31% of the prescribed dose had been administered), the peak plasma asparaginase activity was lower (0.1–0.30 IU/mL), the plasma asparagine levels were still undetectable as long as the asparaginase activity level remained above the threshold of 0.02 IU/mL. Our data suggest that depletion of plasma asparagine was maintained until the plasma asparaginase level fell below 0.02 IU/mL.
Previously published reports have concluded that a reasonable target level of asparaginase activity associated with asparagine depletion have been 0.05–0.4 IU/mL.[5–14] Other reports using non-pegylated asparaginase (E. coli or Erwinia chrysanthemi) have noted that with levels below 0.1 IU/mL, complete asparagine depletion can still be observed although an alternative cutoff was not suggested.[13,18] The FDA used trough plasma asparaginase levels ≥0.1 IU/mL in their approval of Erwinia asparaginase.[15] The prior report of the clinical trial analyzed here described the threshold as <0.4 IU/mL [16] but did not specifically attempt to quantitate the level as presented in this analysis.
There are well known inherent challenges in ex vivo neutralization of asparaginase activity that may have led to ex vivo plasma asparagine depletion in some samples.[19,20] In order to minimize ex vivo deamination (hydrolysis, degradation) of plasma asparagine, the PK/PD samples analyzed were collected with strict on-site instruction and monitoring of the processing of blood samples. These included promptly placing specimens on ice, centrifugation at 4°C, separation of plasma, addition of SERAPREP®, and flash freezing with dry ice and storage at −70°C.[16] Our data demonstrated that this processing was effective at minimizing ex vivo deamination as the two samples which had plasma asparaginase activity between 0.013 and 0.02 IU/ml had plasma asparagine detected in the matched sample.
At lower levels of asparaginase activity, particularly below 0.1 IU/ml, and/or as the temperature declines, ex vivo plasma asparagine depletion is diminished, as shown in data published by Lanvers-Kaminsky et al [20]. In examining a regression of their published data, which had been generated with native E.coli asparaginase, over the 0.001– 0.1 IU/mL range of asparaginase levels, there is more than 50% reduction in ex vivo degradation of asparagine at an asparaginase level of 0.02 IU/mL than at 0.1 IU/mL (Supplemental Figure S3A). Additionally, the km and vmax of pegaspargase as compared to native E.coli asparaginase indicate that pegaspargase provides less ex vivo degradation than the native enzyme and in particular pegaspargase is more substrate dependent than native enzyme. Taken together, one would predict that compared with an aspariginase level of 0.1 IU/mL, a level of 0.02 IU/mL, would have the 80% slower velocity of ex vivo degradation of asparagine (Supplemental Fig. S3B). Thus, the ex vivo hydrolysis of asparagine is less problematic at the lower asparaginase levels that we studied.
Additionally, as asparaginase does not cross the blood brain barrier, CSF asparagine is not at risk for ex vivo degradation of asparagine. Although limited by the number of evaluable matched CSF asparagine/plasma asparaginase pairs, our data support the selection of 0.02 IU/mL as the threshold level for systemic asparagine depletion based on focusing on pairs where the plasma asparaginase levels were between 0.02 and 0.05 IU/mL
The lower limit of the plasma asparaginase assay used in this study was 0.013 IU/mL, within 0.007 IU/mL below the therapeutic drug monitoring threshold level of 0.02 IU/mL suggested by the results of the study. Given the proximity of this threshold level to the lower limit of detection, plasma samples obtained within 43 days after a 2,500 IU/m2 dose of pegaspargase that have “undetectable” plasma asparaginase activity could be considered to have residual, non-measurable plasma asparaginase, especially if the last sample in the patient had a measurable level. By assuming that these samples had residual plasma asparaginase at a specific level, e.g., 1 log lower than the lower limit of assay sensitivity, the levels between 0.02 and 0.05 IU/mL can be more critically assessed for significance and consistency with levels higher and lower than this range. The slope of the linear regression of the 22 paired samples with plasma asparaginase levels between 0.05 and 0.22 IU/mL to lower asparaginase levels (Fig. 4, black symbols) is consistent with the slope of the analogous regression of the 10 paired (CSF/plasma) samples with plasma asparaginase levels between 0.02 and 0.05 IU/mL (Fig. 4, red symbols). This observation is also concordant with 10 paired samples with plasma asparaginase levels between 0.02 and 0.05 IU/mL being within the 95% confidence interval of the 22 paired samples with plasma asparaginase levels above 0.05 IU/mL (Fig. 4, table and as described in Results). This paired level analysis supports the validity of the measurable levels below as the threshold for therapeutic drug monitoring.
While the other chemotherapy agents used in delayed intensification and interim maintenance are similar to those used in induction and consolidation, we only had samples to analyze from induction and consolidation. Importantly, PK/PD did not significantly differ in induction and consolidation. Our PK analysis is consistent with prior reports of accelerated clearance of asparaginase activity beginning 7–14 days after a dose of 2,500 IU/m2 of pegylated asparaginase.[21,22] Our PK/PD results suggest that 96% of children and young adults treated for NCI HR ALL with 2500 IU/m2 of pegaspargase IV have asparagine depletion for 22 to 29 days. The induction and consolidation asparaginase activity profiles were nearly identical in two-thirds of the patients, and one-sixth of the patients had either higher or lower levels for most of the period of plasma asparagine depletion. One patient had evidence a neutralizing antibody and inactivation after exposure to the first dose and this is consistent with prior reports of much lower rates of silent and clinical inactivation occurring with pegaspargase than with native asparaginase.[23,24] Due to limited patient numbers, we were unable to correlate PK/PD with long-term survival.
Recently, Bleyer et al[25] published recommendations for the use of a clinically available (in the US) assay for plasma asparaginase levels for therapeutic drug monitoring following administration of pegaspargase. This algorithm suggests that a 14 day level >0.1 IU/mL is consistent with adequate exposure to asparaginase and that using their algorithm results in an avoidance of unnecessary changes in asparaginase therapy which may contribute to poor outcomes.[25] Our data could be used to optimize appropriate target levels at various intervals after dosing of pegaspargase.
Our data indicate that a level of plasma asparaginase activity of 0.02 IU/mL can deplete plasma asparagine which is considerably lower than the previously reported threshold levels of 0.05 to 0.4 IU/mL.[5–14] These data suggest that more information is needed to define asparaginase dosing regimens used in pediatric ALL therapy in order to optimize both PK values and cure rates.
Supplementary Material
Acknowledgments
Supported by: Supported by grants from National Cancer Institute to the Children’s Oncology Group (U10 CA98543, U10 CA98413, U10 180886, U10 CA180899) and by research funding from Enzon Inc., and Sigma-Tau Pharmaceuticals. SPH is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics, Children’s Hospital of Philadelphia. MLL is the Benioff Chair of Children’s Health and the Ablin Professor of Molecular Oncology in the Department of Pediatrics, Benioff Children’s Hospital, San Francisco, CA.
Trial registration: clinicaltrials.gov identifier NCT00671034
Footnotes
Disclosures: RJS has been on an advisory board and received honoraria from Baxalta Pharmaceuticals and Shire Pharmaceuticals. AB has been on an advisory board and received honoraria from Baxalta Pharmaceuticals, Shire Pharmaceuticals and Jazz Pharmaceuticals. SPH has received honoraria from Jazz Pharmaceuticals, Sigma Tau Pharmaceuticals, Spectrum Pharmaceuticals, and Erytech and consulting fees from Novartis. ALA has been on an advisory board and received honoraria from Shire Pharmaceuticals. MD, GHR, NW, MLL, EAR, and WLC have no COI to disclose.
Presented: In part at the 52nd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3–7, 2016
References
- 1.Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med 2015;373:1541–1552. [DOI] [PubMed] [Google Scholar]
- 2.Asselin B, Rizzari C. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leuk Lymphoma 2015;56:2273–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. II. Lymphoma 6C3HED cells cultured in a medium devoid of L-asparagine lose their susceptibility to the effects of guinea pig serum in vivo. J Exp Med 1963;118:121–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med 1963;118:99–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riccardi R, Holcenberg JS, Glaubiger DL, Wood JH, Poplack DG. L-asparaginase pharmacokinetics and asparagine levels in cerebrospinal fluid of rhesus monkeys and humans. Cancer Res 1981;41:4554–4558. [PubMed] [Google Scholar]
- 6.Ahlke E, Nowak-Göttl U, Schulze-Westhoff P, et al. . Dose reduction of asparaginase under pharmacokinetic and pharmacodynamic control during induction therapy in children with acute lymphoblastic leukaemia. Br J Haematol 1997;96(4):675–681. [DOI] [PubMed] [Google Scholar]
- 7.Albertsen BK, Schroder H, Ingerslev J, et al. . Comparison of intramuscular therapy with Erwinia asparaginase and asparaginase Medac: pharmacokinetics, pharmacodynamics, formation of antibodies and influence on the coagulation system. Br J Haematol 2001;115(4):983–990. [DOI] [PubMed] [Google Scholar]
- 8.Albertsen BK, Schroder H, Jakobsen P, Müller HJ, Carlsen NT, Schmiegelow K. Monitoring of Erwinia asparaginase therapy in childhood ALL in the Nordic countries. Br J Clin Pharmacol 2001;52(4):433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avramis VI, Panosyan EH. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin Pharmacokinet 2005;44(4):367–393. [DOI] [PubMed] [Google Scholar]
- 10.Boos J, Werber G, Ahlke E, et al. . Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur J Cancer 1996;32A(9):1544–1550. [DOI] [PubMed] [Google Scholar]
- 11.Douer D, Yampolsky H, Cohen LJ, et al. . Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adults 55 years old or younger with newly diagnosed acute lymphoblastic leukemia. Blood 2007;109:2744–2750. [DOI] [PubMed] [Google Scholar]
- 12.Rizzari C, Zucchetti M, Conter V, et al. . L-asparagine depletion and L-asparaginase activity in children with acute lymphoblastic leukemia receiving i.m. or i.v. Erwinia C. or E. coli L-asparaginase as first exposure. Ann Oncol 2000;11(2):189–193. [DOI] [PubMed] [Google Scholar]
- 13.Vieira Pinheiro JP, Ahlke E, Nowak-Göttl U, et al. . Pharmacokinetic dose adjustment of Erwinia asparaginase in protocol II of the paediatric ALL/NHL-BFM treatment protocols. Br J Haematol 1999;104(2):313–320. [DOI] [PubMed] [Google Scholar]
- 14.Avramis VI, Sencer S, Periclou AP, et al. . A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood 2002;99:1986–1994. [DOI] [PubMed] [Google Scholar]
- 15.Salzer WL, Asselin B, Supko JG, et al. . Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: a report from the Children’s Oncology Group. Blood 2013;122:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angiolillo AL, Schore RJ, Devidas M, et al. . Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from Children’s Oncology Group Study AALL07P4. J Clin Oncol 2014;32:3874–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith M, Arthur D, Camitta B, et al. . Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol 1996;14:18–24. [DOI] [PubMed] [Google Scholar]
- 18.Klug Albertsen B, Schmiegelow K, Schrøder H, et al. . Anti-Erwinia asparaginase antibodies during treatment of childhood acute lymphoblastic leukemia and their relationship to outcome: a case-control study. Cancer Chemother Pharmacol 2002;50(2):117–120. [DOI] [PubMed] [Google Scholar]
- 19.Asselin BL, Lorenson MY, Whitin JC, et al. . Measurement of serum L-asparagine in the presence of L-asparaginase requires the presence of an L-asparaginase inhibitor. Cancer Res 1991;51:6568–6573. [PubMed] [Google Scholar]
- 20.Lanvers-Kaminsky C, Westhoff PS, D’Incalci M, Zucchetti M, Boos J. Immediate cooling does not prevent the ex vivo hydrolysis of L-asparagine by asparaginase. Ther Drug Monit 2014;36:549–552. [DOI] [PubMed] [Google Scholar]
- 21.Hempel G, Muller HJ, Lanvers-Kaminsky C, Wurthwein G, Hoppe A, Boos J. A population pharmacokinetic model for pegylated-asparaginase in children. Br J Haematol 2010;148:119–125. [DOI] [PubMed] [Google Scholar]
- 22.Muller HJ, Loning L, Horn A, et al. . Pegylated asparaginase (Oncaspar) in children with ALL: drug monitoring in reinduction according to the ALL/NHL-BFM 95 protocols. Br J Haematol 2000;110:379–384. [DOI] [PubMed] [Google Scholar]
- 23.Silverman LBBT, Hunt SK, et al. Randomized Study of Pegaspargase and calaspargase pegol (SC-PEG) in pediatric patients with newly diagnosed acute lymphoblastic leukemia or lymphoblastic lymphoma: Results of DFCI ALL Consortium Protocol 11–001. American Society of Hematology Volume 128:175; no. 22 175 0006–4971; 2016. [Google Scholar]
- 24.Place AE, Stevenson KE, Vrooman LM, et al. . Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05–001): a randomised, open-label phase 3 trial. Lancet Oncol 2015;16:1677–1690. [DOI] [PubMed] [Google Scholar]
- 25.Bleyer A, Asselin BL, Koontz SE, Hunger SP. Clinical application of asparaginase activity levels following treatment with pegaspargase. Pediatr Blood Cancer 2015;62:1102–1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


