Abstract
Aim
To investigate tumour motion tracking uncertainties in the CyberKnife Synchrony system with single fiducial marker in liver tumours.
Background
In the fiducial-based CyberKnife real-time tumour motion tracking system, multiple fiducial markers are generally used to enable translation and rotation corrections during tracking. However, sometimes a single fiducial marker is employed when rotation corrections are not estimated during treatment.
Materials and methods
Data were analysed for 32 patients with liver tumours where one fiducial marker was implanted. Four-dimensional computed tomography (CT) scans were performed to determine the internal target volume (ITV). Before the first treatment fraction, the CT scans were repeated and the marker migration was determined. Log files generated by the Synchrony system were obtained after each treatment and the correlation model errors were calculated. Intra-fractional spine rotations were examined on the spine alignment images before and after each treatment.
Results
The mean (standard deviation) ITV margin was 4.1 (2.3) mm, which correlated weakly with the distance between the fiducial marker and the tumour. The mean migration distance of the marker was 1.5 (0.7) mm. The overall mean correlation model error was 1.03 (0.37) mm in the radial direction. The overall mean spine rotations were 0.27° (0.31), 0.25° (0.22), and 0.23° (0.26) for roll, pitch, and yaw, respectively. The treatment time was moderately associated with the correlation model errors and weakly related to spine rotation in the roll and yaw planes.
Conclusions
More caution and an additional safety margins are required when tracking a single fiducial marker.
Keywords: CyberKnife, Synchrony system, Fiducial marker tracking, Liver tumour
Abbreviations: AP, anterior–posterior; CTV, clinical target volume; GTV, gross tumour volume; LED, light-emitting diode; ITV, internal target volume; LR, left–right; PTV, planning target volume; SBRT, stereotactic body radiation therapy; SD, standard deviation; SI, superior–inferior; XST, Xsight Spine Tracking
1. Background
Stereotactic body radiation therapy (SBRT) can be considered as an alternative treatment for patients with liver cancer who are not eligible for ablation or transcatheter arterial chemoembolisation.1, 2 SBRT delivers high-dose radiation to the tumour via a small number of fractions and minimises radiation-induced liver damage. The role of SBRT in the treatment of hepatocellular carcinoma and metastatic tumour has been the subject of several recent reviews, all of which show that SBRT has promising results with a low risk of serious toxicity.2, 3, 4 Management of respiration-related tumour motion, such as respiratory gating and real-time tumour tracking, are applied in SBRT to reduce the volume of the surrounding tissues that might be irradiated.5, 6 Implantation of internal fiducial markers in the liver has become the clinical standard because it is an effective and safe procedure that allows the tumour position to be tracked.7 During treatment, intra-fractional kV X-ray images can be used to verify the positions of these fiducial markers for tumour tracking.8 The fiducial markers can also improve the set-up accuracy when compared with the bony anatomy set-up.9
The CyberKnife Synchrony Respiratory Tracking System (Accuray Inc., Sunnyvale, CA, USA) can be used for delivery of SBRT with real-time tumour motion tracking to sub-millimetre accuracy,5, 10, 11 which allows for the delivery of high-dose radiation to a tumour in a few fractions. Previous reports on patients with liver metastases treated by CyberKnife have demonstrated excellent local control rates with very low toxicity.4 The system has two orthogonal diagnostic X-ray sources fixed in the ceiling and flat-panel detectors under the floor to image internal targets, minimising errors caused by intra-fractional patient motion. The respiratory motion of a patient is monitored by a camera array mounted onto the ceiling and light-emitting diode (LED) markers attached to the patient. A correlation model for the determination of the relationship between the external LED marker positions and internal target positions is also used for real-time tracking.12, 13 Typically, gold markers are placed near or within the tumour to act as internal targets for prediction of the position of the tumour, and more than three usable fiducial markers are required to allow for six-directional corrections (translations and rotations) during treatment.14, 15
In clinical practice, it is possible to track a tumour with fewer than two fiducial markers without risking complications such as haemorrhage and a high number of artefacts on computed tomography (CT) images, while avoiding the complexity associated with the use of multiple fiducial markers during tracking.12, 16 In this situation, patients are aligned to the planned position by referencing the spinal structures near the fiducial markers with translational and rotational corrections at the start of the treatment. Translational corrections are only possible during beam delivery. Therefore, some uncertainties related to tracking without rotational corrections during treatment are needed to be investigated because CyberKnife SBRT localises the target very precisely and the treatment time is longer than that of typical external beam radiation therapy. Although many studies have demonstrated the accuracy of normal multi-fiducial tracking with the CyberKnife Synchrony system,12, 17, 18 there has been no in-depth investigation of the uncertainties of tracking with fewer than two fiducial markers. It is also unclear if this tracking method has the same accuracy as a normal multi-fiducial tracking system. It is important to assess the appropriate margins in the treatment plan when fewer than two fiducial markers are used for tracking.
In this study, we retrospectively analysed the following factors in patients with liver tumours treated at our institution using the CyberKnife Synchrony system with only a single fiducial marker: internal target volume (ITV) margin, migration of the marker, correlation model error, and intra-fractional spine rotations during beam delivery. This study might contribute to the management of treatment for liver cancer using a single fiducial tracking method.
2. Materials and methods
2.1. Patients and treatment plan
Data from 32 patients with liver tumours were retrospectively analysed. These patients were treated at Kobe Minimally Invasive Cancer Center (Hyogo, Japan) using SBRT based on CyberKnife Synchrony Respiratory Tracking System (VSI, version 9.6.0) on a six-directional moving couch between October 2016 and March 2018 were retrospectively analysed. The study data were restricted to be only from patients who showed no visible movement during treatment. One tumour for each patient was treated in four fractions with individually tailored doses varying between 40 Gy and 60 Gy. One Gold Anchor fiducial marker (Naslund Medical AB, Huddinge, Sweden) was implanted percutaneously near the tumour under ultrasound guidance in each patient. Approximately one week after placement of the fiducial marker, 1.0-mm-thick CT slices were collected from patients during breath-hold in expiration for treatment planning. The patients were immobilised in the supine position with arms raised using full-body vacuum cushions (Vac-Lok, CIVCO Medical Solutions, Coralville, IA, USA). One patient was positioned with the arms at the sides. The patient characteristics are shown in Table 1. The ethics approval for this study was obtained from the appropriate committee at our institution (reference 2017-kenkyu11-08).
Table 1.
Patient characteristics.
| Characteristics | All plans (n = 32) |
|---|---|
| Sex | |
| Male | 20 (62.5%) |
| Female | 12 (37.5%) |
| Age (years) | |
| Median (range) | 69.5 (51–85) |
| Tumour type | |
| Hepatocellular carcinoma | 21 (65.6%) |
| Metastasis | 11 (34.4%) |
| Tumour site | |
| S2/S3/S4/S5/S6/S7/S6 and 7/S7 and 8/S8 | 2/2/7/1/1/6/2/1/10 |
| Prescribed dose | |
| 60 Gy | 7 (21.9%) |
| 50 Gy | 22 (68.8%) |
| Other | 3 (9.3%) |
| Beams delivered, n | |
| Mean (range) | 58 (31–100) |
Treatment was designed using a MultiPlan system (version 4.6.0; Accuray Inc.). For delineation, contrast-enhanced CT and magnetic resonance images were used to acquire the gross tumour volume (GTV); the clinical target volume (CTV) was equal to the GTV. To account for deformation of the tumour, including its rotation induced by respiratory motion and the motion difference between the fiducial marker and the tumour, each phase of the four-dimensional CT scans was registered to the primary CT images by referencing the fiducial marker on Velocity software (version 3.2.1; Varian Medical System, Palo Alto, CA, USA). The ITV was then created by summation of all the CTV phases. In this study, the size of the ITV margin was defined as the maximum displacement of the original CTV to cover all CTV phases. The amplitude of the respiratory-induced motion of the fiducial marker was also evaluated using the CT data obtained in the expiratory and inspiratory phases. The planning target volume (PTV) was derived via the ITV using 3–4-mm margins, which are routinely used in SBRT by CyberKnife for liver tumours at our centre. Separate from the beam delivery plan, the Xsight Spine Tracking (XST) plan was designed for patient alignment. In this plan, the spinal structure closest to the fiducial marker is set as the alignment centre. The distances from the fiducial marker to the tumour and to the spine alignment centre were obtained from the primary CT images. The distance from the marker to the tumour was measured as the distance between the marker and the edge of the GTV that was closest to the marker. All treatment plans were designed using one or two fixed-size collimators with isocentric or nonisocentric delivery.
2.2. Treatment using tracking of a single fiducial marker
As part of our routine clinical practice, a CT scan was performed in each patient immediately before the first fraction of treatment to assess migration of the marker. The scan was performed in the same way as for treatment planning. Using the Velocity software, the images were then registered to the original planning images by referencing the tumour, and the magnitude of marker migration was measured.
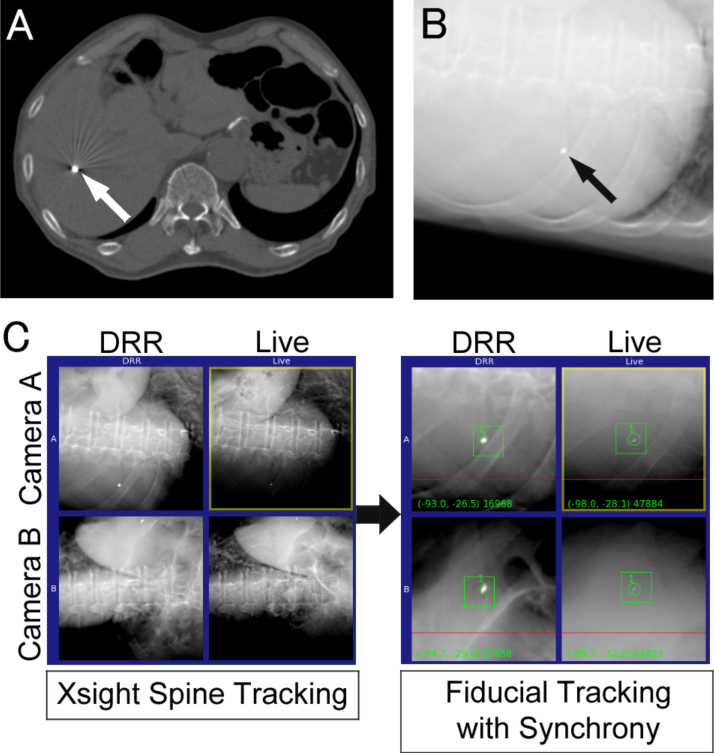
At the beginning of treatment, the XST system was used to acquire the pre-treatment diagnostic images (pre-images) to precisely align the translation and rotation according to the spinal structures near the fiducial marker. The couch was shifted in translation from the spine alignment centre to the centre of the fiducial marker. A respiratory motion model correlated between the LED markers and fiducial positions was then constructed for delivery of real-time tumour tracking by the Synchrony system, based on the images acquired during the various respiratory phases. During beam delivery, new images were acquired at a time interval of 30–60 s and the correlation model was continuously updated. The treatment schema is shown in Fig. 1. Two radiation technologists carefully monitored patient movement and respiratory motion during treatment.
Fig. 1.
An example of single fiducial tracking. (A) Computed tomography image and (B) digitally reconstructed radiograph (DRR) of an inserted fiducial marker (arrowhead). (C) Left image represents Xsight Spine Tracking at the beginning of treatment, and the right image represents the fiducial tracking with the Synchrony system during beam delivery. The upper and lower images show the projections of two orthogonal X-ray sources (cameras A and B).
2.3. Correlation model error
Data in ModelPoint.log, which is the log data file produced by the Synchrony system, was obtained after each treatment fraction in each patient to analyse the correlation model error, as described previously.13 The data in this file were recorded at the time of each image acquisition during treatment. The correlation model error was defined as the difference between a tumour location as determined by images and that calculated by the latest correlation model. The overall mean error of each fraction was calculated for each patient for the superior–inferior (SI), left–right (LR), and anterior–posterior (AP) directions. Radial errors were calculated by root-squared summation of each direction. The treatment time per fraction was also obtained from the log file and defined as the interval between the first XST alignment and the end of beam delivery.
2.4. Intra-fractional spine rotation
To confirm the intra-fractional spine rotation during treatment, after the beam delivery was completed, the couch was shifted translationally to match the position of the spine alignment centre of the pre-images and the post-treatment images (post-images) were acquired. The rotational offset values for the pre-images and post-images of each patient were stored in log files produced by the CyberKnife target locating system after each treatment fraction. The intra-fractional spine rotations were then calculated by subtracting the values of the post-images from those of the pre-images for each matrix (along the roll, pitch, and yaw axes). Assuming that the spine rotation was linearly correlated with the target position, the errors of spine rotations on the target position were calculated by subtracting the position vector of the predicted target from that of the rotated target for each axis. The target position was considered as the centre of the PTV. The precision of the values recorded in the log files for the roll component was 0.1 degrees, and that for the pitch and yaw components was less than 0.01 degrees.
2.5. Statistical analysis
The data are calculated for each fraction and are expressed as the overall mean ± standard deviation for all patients. The Pearson correlation coefficient (r) was evaluated with a test of no correlation; the 95% confidence level (p < 0.05) was considered significant. The statistical analyses were performed using EZR software (Saitama Medical Centre, Jichi Medical University, Saitama, Japan).19
3. Results
The data for 32 treatment plans were analysed. As shown in Table 2, the mean GTV and ITV values were 9.0 ± 8.3 cm3 and 11.6 ± 9.9 cm3, respectively. The overall mean motion amplitude of the fiducial marker was 13.1 ± 5.4 mm in the radial direction. The mean distances from the fiducial marker to the tumour and to the spine alignment centre in the radial direction were 15.6 ± 15.2 mm and 9.3 ± 1.8 cm, respectively. The mean of the ITV margin on the treatment plans was 4.1 ± 2.3 mm and was weakly correlated with the distance between the fiducial marker and the tumour (r = 0.35; p < 0.05). There were no significant correlations between the ITV margins and the GTV volumes or the marker motion amplitude. The CT scans performed prior to the treatment did not reveal any cases of gross migration of the fiducial markers. The overall mean measured migration was 1.5 ± 0.7 mm, and the 95th percentile was 2.7 mm. Furthermore, no fractions revealed any visible patient movement, treatment interruption, or realignment of the spine during beam delivery. The mean treatment time per fraction was 32.3 ± 8.4 min.
Table 2.
Summary of treatment parameters.
| Parameters | Mean | SD | Range |
|---|---|---|---|
| GTV volume (cm3) | 9.0 | 8.3 | 0.6–33.5 |
| ITV volume (cm3) | 11.6 | 9.9 | 1.2–38.1 |
| Marker motion amplitude (mm) | |||
| SI | 12.2 | 5.2 | 2.0–24.0 |
| LR | 1.5 | 1.0 | 0.0–4.4 |
| AP | 4.2 | 2.5 | 1.0–10.6 |
| Radial | 13.1 | 5.4 | 2.7–26.3 |
| Marker–GTV distance (mm) | |||
| SI | 4.9 | 5.7 | 0.0–25.2 |
| LR | 8.4 | 11.6 | 0.0–49.0 |
| AP | 8.9 | 11.5 | 0.0–41.4 |
| Radial | 15.6 | 15.2 | 0.0–55.4 |
| Marker–XST centre distance (cm) | |||
| SI | 0.5 | 0.6 | 0.0–2.2 |
| LR | 5.5 | 3.0 | 0.4–9.7 |
| AP | 6.2 | 3.6 | 0.5–12.0 |
| Radial | 9.3 | 1.8 | 5.3–12.4 |
| ITV margin (mm) | 4.1 | 2.3 | 0.5–9.1 |
| Marker migration (mm) | 1.5 | 0.7 | 0.2–3.3 |
| Treatment time per fraction (min) | 32.3 | 8.4 | 15–66 |
AP, anterior–posterior; GTV, gross tumour volume; ITV, internal target volume; LR, left–right; SD, standard deviation; SI, superior–inferior; XST, Xsight Spine Tracking.
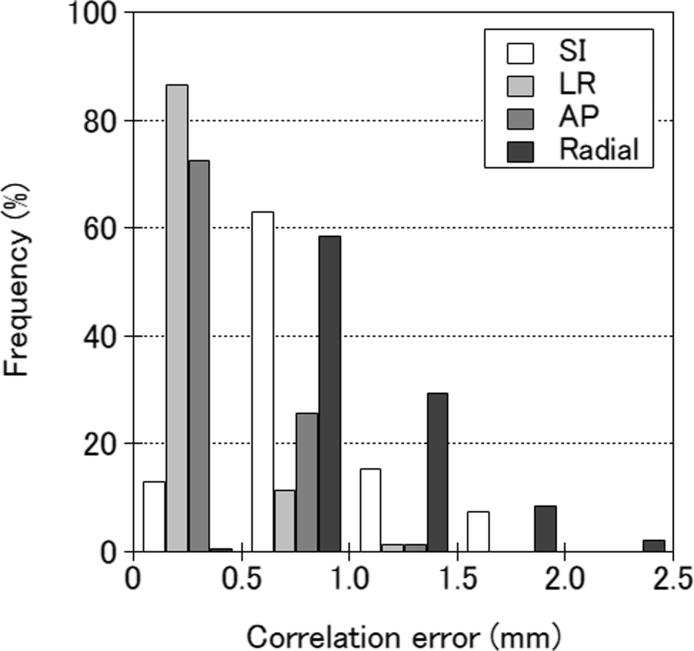
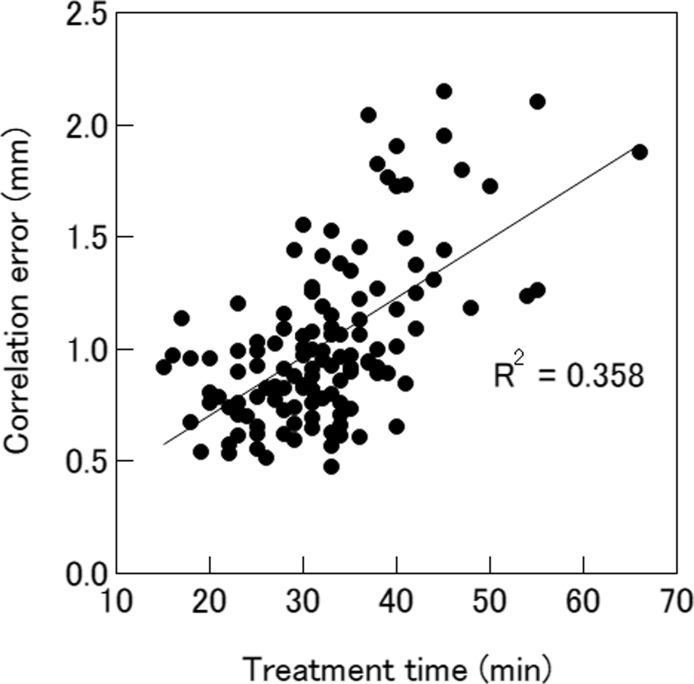
The histograms of the correlation model errors for overall fractions, analysed for 128 fractions from 32 patients, are shown in Fig. 2. The mean correlation error was 0.83 ± 0.35 mm, 0.36 ± 0.18 mm, 0.44 ± 0.18 mm, and 1.03 ± 0.37 mm in the SI, LR, AP, and radial directions, respectively. The correlation errors for the radial direction were moderately correlated with treatment time (r = 0.60; p < 0.01), as shown in Fig. 3. There were no significant correlations between the errors and the marker motion amplitude or the distance between the fiducial marker and the spine alignment centre.
Fig. 2.
Distribution of the mean correlation errors for the superior–inferior (SI), left–right (LR), anterior–posterior (AP), and three-dimensional radial directions for 128 fractions.
Fig. 3.
Correlation model errors as functions of the treatment time. The lines are obtained via the least squares fit method.
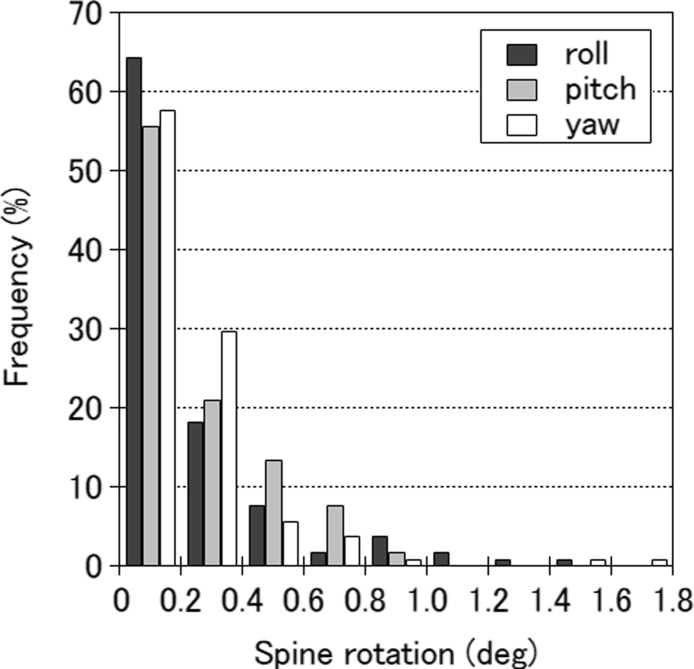
Intra-fractional spine rotation data obtained from 104 fractions in 26 patients were analysed. Fig. 4 shows the histograms of the spine rotational errors for each matrix. The overall mean rotation errors were 0.27 ± 0.31°, 0.25 ± 0.22°, and 0.23 ± 0.26° for roll, pitch, and yaw, respectively. The rotational errors for roll and yaw were found to be weakly correlated with treatment time (r = 0.23 and r = 0.24, respectively; p < 0.05). The errors of these spine rotations on the target position were estimated based on the position relative to the spine, fiducial marker, and PTV. The 95th percentile error was 0.35 mm, 0.33 mm, and 0.22 mm in the roll, pitch, and yaw planes, respectively.
Fig. 4.
Distribution of the mean intra-fractional rotational spine errors for the roll, pitch, and yaw of 104 fractions.
4. Discussion
In this study, we assessed the global uncertainties of treatment of liver tumours using a single fiducial-based CyberKnife Synchrony system. To our knowledge, there have been no studies documented in the literature focused on tracking of a single fiducial marker. Therefore, our results provide valuable and useful information regarding appropriate treatment margins when using single fiducial tracking methods.
We evaluated the model correlation errors during treatment of liver tumours using single fiducial-based tracking. There have been some reports on correlation errors when the CyberKnife Synchrony system is used to treat liver tumours.12, 20, 21 Winter et al. investigated the correlation errors in case of patients with liver cancer who had 1–6 fiducial markers used for tracking. They showed that the overall mean error to be 1.7 ± 1.1 mm in the radial direction.12 Our figure of 1.1 ± 0.4 mm, which is estimated from tracking of only a single fiducial marker, is similar to that in their report. However, a study by Chan et al., who investigated 24 patients with liver metastases treated by CyberKnife Synchrony tracking using more than 4 fiducial markers, found that the mean radial correlation error was 0.4 mm,20 which is considerably smaller than the figure obtained in our study. Although differences in system software versions, fiducial markers, and treatment procedures used may have effect on the results of these studies, it seems likely that single fiducial tracking is more prone to model error than multiple fiducial tracking. Moreover, there is a significant relationship between the correlation model error and the treatment time as determined through our study. This finding is anticipated due to the fact that in the case of single fiducial tracking only one marker is directly affected by a change in respiratory motion during prolonged treatment without rotation corrections. With regard to target motion amplitude, the correlation errors had no significant relationship with the marker motion amplitude. However, in contrast with our results, Winter et al. reported that correlation errors were weakly related to the target amplitude in multiple fiducial tracking for liver cancer.12 The number of fiducial markers used for tracking and analysis of the motion amplitude may explain this discrepancy.
Our results of intra-fractional spine rotations concur with those documented in previous reports that investigated intra-fractional motion of the spine during spinal treatments.22, 23, 24 For example, Fürweger et al. analysed patient movement during spinal treatment with a CyberKnife XST system and found that the mean rotational errors were 0.40 ± 0.20°, 0.20 ± 0.08°, and 0.19 ± 0.08° for roll, pitch, and yaw, respectively.22 The errors for roll and yaw were weakly correlated with the treatment time and indicated a trend of a slight increase in the error value with increasing treatment time. This suggests that the relatively long treatment time per fraction of CyberKnife SBRT may increase intra-fractional rotation errors. Moreover, our results agree with those reported by Hoogeman et al. who demonstrated that the rotational random error occurring during CyberKnife treatment was only slightly dependent on the treatment time.25 They also recommended that the patient position and orientation be checked at least every 5 min during the treatment to ensure high-precision treatment delivery. Although only images before and after treatment were evaluated in our study, it can be concluded that using the XST system to acquire images at regular time intervals and perform rotational spine realignments enables minimisation of intra-fractional spine rotation errors for single fiducial marker tracking. However, as a result of a discontinuous operation of the Synchrony system and reconstruction of respiratory correlation models longer treatment times are experienced. We also found that the errors caused by spine rotations on the target position were relatively small; the 95th percentile errors were less than 0.4 mm. Bertholet et al. demonstrated that the marker-based set-up accuracy with rotational correction based on bony anatomy was the same as that without rotational correction.9 These results suggest that the intra-fractional spine rotations observed using the single fiducial tracking method had minimal impact on the total targeting errors. It should be noted that spine realignments are necessary for single marker tracking to confirm rotational errors when patient movement is detected visually during beam delivery.
The impact of rotation by breath motion on overall intra-fractional rotational errors during liver treatment seems to be more significant than that of spine rotation.20, 26 Xu et al. evaluated tumour motion in the case of multiple fiducial markers and demonstrated that the mean intra-fractional rotation of the roll, pitch, and yaw angles was 1.2 ± 1.8°, 1.8 ± 2.4°, and 1.7 ± 2.1°, respectively.27 Their data include rotational errors other than those related to the spine, such as the liver and tumour rotations caused by breath-related movement; therefore, their error values are somewhat larger than ours, which only include the cause of the spine rotation. Additional ITV margins are needed for single fiducial tracking to account for tumour rotation and deformation by breath motion. Our data show that the mean ITV margin size of 4.1 mm used for single fiducial tracking was weakly correlated with the distance between the marker and the tumour. This indicates that placement of the marker directly affects the margin size in single fiducial tracking and is more crucial than when using the multi-fiducial tracking method. Even when multi-fiducial markers are used, an increasing distance between the fiducial markers and the tumour reduces the accuracy of a marker-guided set-up.15 Unlike with multi-fiducial tracking, the ITV margin used routinely for single fiducial marker tracking in our clinical practice could not completely compensate for the intra-fractional rotation errors caused by a change in the breath motion pattern. Liang et al. reported significant variability in intra-fractional liver motion amplitude when performing SBRT using a CyberKnife in patients with liver tumours.28 The main limitation of this study is that the impact of variation in motion of the tumour and liver on rotation errors was not assessed because only a single marker was used. Further clinical studies are required to confirm the overall targeting accuracy of single fiducial tracking methods.
Migration of the fiducial marker when using a single marker tracking method should be carefully monitored. Re-implantation must be considered in the case of fiducial marker displacement before treatment, which delays treatment. There are mixed reports in the literature regarding migration of liver implanted fiducial markers; some reported non-migration16, 29, while others reported existing migration of such markers through the vena cava.30, 31 Furthermore, even if there is no gross migration, marker displacement in the order of few millimetres has been reported.31, 32 In this study, we analysed CT images that were acquired immediately before delivery of the first fraction of treatment and found the 95th percentile of migration distance to be about 2.7 mm when compared with the planning CT images. Although such small displacements might include the CT uncertainties about the errors introduced by the software and the reproducibility of the marker position for respiratory motion,8 they may lead directly to targeting errors because tumour prediction is completely dependent on one fiducial marker.15 Therefore, this uncertainty should be included in the PTV margin to account for possible migration of the maker. Moreover, the manufacturer of the CyberKnife system has acknowledged that single fiducial tracking increases the risk of beam mis-targeting.
5. Conclusions
Single fiducial marker tracking for CyberKnife treatment of the liver can be adequately used in the case of non-availability of multi-fiducial markers. However, compared with the multi-fiducial tracking method, additional safety margins are required for this treatment. Our results show that the mean CTV–ITV margin of 4.1 mm was used to account for the overall uncertainties caused by respiratory motion. The size of the ITV may be affected by the distance between the fiducial marker and the tumour. The uncertainty of 3 mm or less is reflected for the final PTV margin to compensate for migration of the marker and intra-fractional spine rotation. The treatment time can be one of the crucial factors affecting the target accuracy in case of single fiducial tracking. Careful management of marker migration and variability in respiratory motion for each patient is essential during the treatment period.
Authors’ contributions
MN, HN, and RS contributed to the concept and design of the study. MN, ST, and YM acquired the patient data. MN and KU analysed the data. MN, HN, and HM participated in the analysis and interpretation of the data. ST, NM, and RS contributed administrative, technical, and statistical support. MN and MG contributed to the writing of the manuscript. All of the authors read and approved the final draft of the manuscript.
Conflict of interest
None declared.
Financial disclosure
None declared.
Contributor Information
Masao Nakayama, Email: naka2008@med.kobe-u.ac.jp.
Kazuyuki Uehara, Email: uehara@k-mcc.net.
Hideki Nishimura, Email: westvill@med.kobe-u.ac.jp.
Shuhei Tamura, Email: cp3ai3dw3@yahoo.co.jp.
Yoshiki Munetomo, Email: munetomo@k-mcc.net.
Shinji Tsudou, Email: tsudou@hyogo-cc.jp.
Hiroshi Mayahara, Email: mayahara@k-mcc.net.
Naritoshi Mukumoto, Email: nmukumot@med.kobe-u.ac.jp.
Moshi Geso, Email: moshi.geso@rmit.edu.au.
Ryohei Sasaki, Email: rsasaki@med.kobe-u.ac.jp.
References
- 1.Bujold A., Massey C.A., Kim J.J. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 2.Haddad M.M., Merrell K.W., Hallemeier C.L. Stereotactic body radiation therapy of liver tumors: post-treatment appearances and evaluation of treatment response: a pictorial review. Abdom Radiol. 2016;41:2061–2077. doi: 10.1007/s00261-016-0768-x. [DOI] [PubMed] [Google Scholar]
- 3.Comito T., Clerici E., Tozzi A., D’Agostino G. Liver metastases and SBRT: a new paradigm? Rep Pract Oncol Radiother. 2015;20:464–471. doi: 10.1016/j.rpor.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ihnát P., Skácelíková E., Tesař M., Penka I. Stereotactic body radiotherapy using the CyberKnife® system in the treatment of patients with liver metastases: state of the art. Onco Targets Ther. 2018;11:4685–4691. doi: 10.2147/OTT.S165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schweikard A., Shiomi H., Adler J. Respiration tracking in radiosurgery. Med Phys. 2004;31:2738–2741. doi: 10.1118/1.1774132. [DOI] [PubMed] [Google Scholar]
- 6.Keall P.J., Cattell H., Pokhrel D. Geometric accuracy of a real-time target tracking system with dynamic multileaf collimator tracking system. Int J Radiat Oncol Biol Phys. 2006;65:1579–1584. doi: 10.1016/j.ijrobp.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Park S.H., Won H.J., Kim S.Y. Efficacy and safety of ultrasound-guided implantation of fiducial markers in the liver for stereotactic body radiation therapy. PLoS One. 2017:e0179676. doi: 10.1371/journal.pone.0179676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedos L., Riou O., Aillères N. Evaluation of reproducibility of tumor repositioning during multiple breathing cycles for liver stereotactic body radiotherapy treatment. Rep Pract Oncol Radiother. 2017;22:132–140. doi: 10.1016/j.rpor.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertholet J., Worm E., Høyer M., Poulsen P. Cone beam CT-based set-up strategies with and without rotational correction for stereotactic body radiation therapy in the liver. Acta Oncol. 2017;56:860–866. doi: 10.1080/0284186X.2017.1288925. [DOI] [PubMed] [Google Scholar]
- 10.Dieterich S., Cavedon C., Chuang C.F. Report of AAPM TG 135: quality assurance for robotic radiosurgery. Med Phys. 2011;38:2914–2936. doi: 10.1118/1.3579139. [DOI] [PubMed] [Google Scholar]
- 11.Ozhasoglu C., Saw C.B., Chen H. Synchrony—Cyberknife respiratory compensation technology. Med Dosim. 2008;33:117–123. doi: 10.1016/j.meddos.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Winter J.D., Wong R., Swaminath A., Chow T. Accuracy of robotic radiosurgical liver treatment throughout the respiratory cycle. Int J Radiat Oncol Biol Phys. 2015;93:916–924. doi: 10.1016/j.ijrobp.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama M., Nishimura H., Mayahara H. Clinical log data analysis for assessing the accuracy of the CyberKnife fiducial-free lung tumor tracking system. Pract Radiat Oncol. 2018;8:e63–e70. doi: 10.1016/j.prro.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Kilby W., Dooley J.R., Kuduvalli G., Sayeh S., Maurer C.R., Jr. The CyberKnife robotic radiosurgery system in 2010. Technol Cancer Res Treat. 2010;9:433–452. doi: 10.1177/153303461000900502. [DOI] [PubMed] [Google Scholar]
- 15.Seppenwoolde Y., Wunderink W., Wunderink-van Veen S.R., Storchi P., Méndez Romero A., Heijmen B.J. Treatment precision of image-guided liver SBRT using implanted fiducial markers depends on marker-tumour distance. Phys Med Biol. 2011;56:5445–5468. doi: 10.1088/0031-9155/56/17/001. [DOI] [PubMed] [Google Scholar]
- 16.Ohta K., Shimohira M., Murai T. Percutaneous fiducial marker placement prior to stereotactic body radiotherapy for malignant liver tumors: an initial experience. J Radiat Res. 2016;57:174–177. doi: 10.1093/jrr/rrv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoogeman M., Prévost J.B., Nuyttens J., Pöll J., Levendag P., Heijmen B. Clinical accuracy of the respiratory tumor tracking system of the cyberknife: assessment by analysis of log files. Int J Radiat Oncol Biol Phys. 2009;74:297–303. doi: 10.1016/j.ijrobp.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 18.Jung J., Song S.Y., Yoon S.M. Verification of accuracy of cyberknife tumor-tracking radiation therapy using patient-specific lung phantoms. Int J Radiat Oncol Biol Phys. 2015;92:745–753. doi: 10.1016/j.ijrobp.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 19.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan M., Grehn M., Cremers F. Dosimetric implications of residual tracking errors during robotic SBRT of liver metastases. Int J Radiat Oncol Biol Phys. 2017;97:839–848. doi: 10.1016/j.ijrobp.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 21.Malinowski K.T., McAvoy T.J., George R., Dieterich S., D'Souza W.D. Mitigating errors in external respiratory surrogate-based models of tumor position. Int J Radiat Oncol Biol Phys. 2012;82:e709–e716. doi: 10.1016/j.ijrobp.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fürweger C., Drexler C., Kufeld M., Muacevic A., Wowra B., Schlaefer A. Patient motion and targeting accuracy in robotic spinal radiosurgery: 260 single-fraction fiducial-free cases. Int J Radiat Oncol Biol Phys. 2010;78:937–945. doi: 10.1016/j.ijrobp.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 23.Han Z., Bondeson J.C., Lewis J.H. Evaluation of initial setup accuracy and intrafraction motion for spine stereotactic body radiation therapy using stereotactic body frames. Pract Radiat Oncol. 2016;6:e17–e24. doi: 10.1016/j.prro.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Monserrate A., Zussman B., Ozpinar A., Niranjan A., Flickinger J.C., Gerszten P.C. Stereotactic radiosurgery for intradural spine tumors using cone-beam CT image guidance. Neurosurg Focus. 2017;42:E11. doi: 10.3171/2016.9.FOCUS16356. [DOI] [PubMed] [Google Scholar]
- 25.Hoogeman M.S., Nuyttens J.J., Levendag P.C., Heijmen B.J. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys. 2008;70:609–618. doi: 10.1016/j.ijrobp.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 26.Bertholet J., Worm E.S., Fledelius W., Høyer M., Poulsen P.R. Time-resolved intrafraction target translations and rotations during stereotactic liver radiation therapy: implications for marker-based localization accuracy. Int J Radiat Oncol Biol Phys. 2016;95:802–809. doi: 10.1016/j.ijrobp.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q., Hanna G., Grimm J. Quantifying rigid and nonrigid motion of liver tumors during stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2014;90:94–101. doi: 10.1016/j.ijrobp.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Liang Z., Liu H., Xue J. Evaluation of the intra- and interfractional tumor motion and variability by fiducial-based real-time tracking in liver stereotactic body radiation therapy. J Appl Clin Med Phys. 2018;19:94–100. doi: 10.1002/acm2.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kothary N., Heit J.J., Louie J.D. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vasc Interv Radiol. 2009;20:235–239. doi: 10.1016/j.jvir.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Shirato H., Harada T., Harabayashi T. Feasibility of insertion/implantation of 2.0-mm-diameter gold internal fiducial markers for precise setup and real-time tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:240–247. doi: 10.1016/s0360-3016(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 31.Worm E.S., Bertholet J., Høyer M. Fiducial marker guided stereotactic liver radiotherapy: is a time delay between marker implantation and planning CT needed? Radiother Oncol. 2016;121:75–78. doi: 10.1016/j.radonc.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura K., Shirato H., Shimizu S. Registration accuracy and possible migration of internal fiducial gold marker implanted in prostate and liver treated with real-time tumor-tracking radiation therapy (RTRT) Radiother Oncol. 2002;62:275–281. doi: 10.1016/s0167-8140(02)00017-8. [DOI] [PubMed] [Google Scholar]